Rapid assembly of complex cyclopentanes employing chiral, α,β-unsaturated acylammonium intermediates
- PMID: 24256870
- PMCID: PMC5548179
- DOI: 10.1038/nchem.1788
Rapid assembly of complex cyclopentanes employing chiral, α,β-unsaturated acylammonium intermediates
Abstract
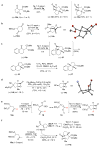
With the intention of improving synthetic efficiency, organic chemists have turned to bioinspired organocascade or domino processes that generate multiple bonds and stereocentres in a single operation. However, despite the great importance of substituted cyclopentanes, given their prevalence in complex natural products and pharmaceutical agents, the rapid, enantioselective assembly of these carbocycles lags behind cyclohexanes. Here, we describe a Michael-aldol-β-lactonization organocascade process for the synthesis of complex cyclopentanes utilizing chiral α,β-unsaturated acylammonium intermediates, readily generated by activation of commodity unsaturated acid chlorides with chiral isothiourea catalysts. This efficient methodology enables the construction of two C-C bonds, one C-O bond, two rings and up to three contiguous stereogenic centres delivering complex cyclopentanes with high levels of relative and absolute stereocontrol. Our results suggest that α,β-unsaturated acylammonium intermediates have broad utility for the design of organocascade and multicomponent processes, with the latter demonstrated by a Michael-Michael-aldol-β-lactonization.
Figures


References
-
- Tietze LF, Brasche G, Gericke KM. Domino reactions in organic synthesis. Wiley-VCH; Weinheim: 2006.
-
- Pellissier H. Stereocontrolled domino reactions. Chem Rev. 2013;113:442–524. - PubMed
-
- Zhou J. Recent Advances in multicatalyst promoted asymmetric tandem reactions. Chem Asian J. 2010;5:422–434. - PubMed
-
- Grondal C, Jeanty M, Enders D. Organocatalytic cascade reactions as a new tool in total synthesis. Nat Chem. 2010;2:167–178. - PubMed
Publication types
MeSH terms
Substances
Associated data
- PubChem-Substance/164178426
- PubChem-Substance/164178427
- PubChem-Substance/164178428
- PubChem-Substance/164178429
- PubChem-Substance/164178430
- PubChem-Substance/164178431
- PubChem-Substance/164178432
- PubChem-Substance/164178433
- PubChem-Substance/164178434
- PubChem-Substance/164178435
- PubChem-Substance/164178436
- PubChem-Substance/164178437
- PubChem-Substance/164178438
- PubChem-Substance/164178439
- PubChem-Substance/164178440
- PubChem-Substance/164178441
- PubChem-Substance/164178442
- PubChem-Substance/164178443
- PubChem-Substance/164178444
- PubChem-Substance/164178445
- PubChem-Substance/164178446
- PubChem-Substance/164178447
- PubChem-Substance/164178448
- PubChem-Substance/164178449
- PubChem-Substance/164178450
- PubChem-Substance/164178451
- PubChem-Substance/164178452
- PubChem-Substance/164178453
- PubChem-Substance/164178454
- PubChem-Substance/164178455
- PubChem-Substance/164178456
- PubChem-Substance/164178457
- PubChem-Substance/164178458
- PubChem-Substance/164178459
- PubChem-Substance/164178460
- PubChem-Substance/164178461
- PubChem-Substance/164178462
- PubChem-Substance/164178463
- PubChem-Substance/164178464
- PubChem-Substance/164178465
- PubChem-Substance/164178466
- PubChem-Substance/164178467
- PubChem-Substance/164178468
- PubChem-Substance/164178469
- PubChem-Substance/164178470
- PubChem-Substance/164178471
- PubChem-Substance/164178472
- PubChem-Substance/164178473
- PubChem-Substance/164178474
- PubChem-Substance/164178475
- PubChem-Substance/164178476
- PubChem-Substance/164178477
- PubChem-Substance/164178478
- PubChem-Substance/164178479
- PubChem-Substance/164178480
- PubChem-Substance/164178481
- PubChem-Substance/164178482
- PubChem-Substance/164178483
- PubChem-Substance/164178484
- PubChem-Substance/164178485
- PubChem-Substance/164178486
- PubChem-Substance/164178487
- PubChem-Substance/164178488
- PubChem-Substance/164178489
- PubChem-Substance/164178490
- PubChem-Substance/164178491
- PubChem-Substance/164178492
- PubChem-Substance/164178493
- PubChem-Substance/164178494
- PubChem-Substance/164178495
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

