Cell-cycle-dependent colonization of mouse spermatogonial stem cells after transplantation into seminiferous tubules
- PMID: 24256919
- PMCID: PMC3958584
- DOI: 10.1262/jrd.2013-083
Cell-cycle-dependent colonization of mouse spermatogonial stem cells after transplantation into seminiferous tubules
Abstract
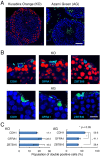
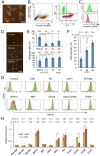
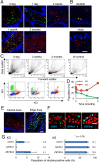
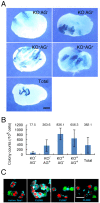
Spermatogonial stem cells (SSCs) migrate to the niche upon introduction into the seminiferous tubules of the testis of infertile animals. However, only 5-10% of the transplanted cells colonize recipient testes. In this study, we analyzed the impact of cell cycle on spermatogonial transplantation. We used fluorescent ubiquitination-based cell cycle indicator transgenic mice to examine the influence of cell cycle on SSC activity of mouse germline stem (GS) cells, a population of cultured spermatogonia enriched for SSCs. GS cells in the G1 phase are more efficient than those in the S/G2-M phase in colonizing the seminiferous tubules of adult mice. Cells in the G1 phase not only showed higher expression levels of GFRA1, a component of the GDNF self-renewal factor receptor, but also adhered more efficiently to laminin-coated plates. Furthermore, this cell cycle-dependency was not observed when cells were transplanted into immature pup recipients, which do not have the blood-testis barrier (BTB) between Sertoli cells, suggesting that cells in the G1 phase may passage through the BTB more readily than cells in the S/G2-M phase. Thus cell cycle status is an important factor in regulating SSC migration to the niche.
Figures
Similar articles
-
Spermatogonial stem cell transplantation into nonablated mouse recipient testes.Stem Cell Reports. 2021 Jul 13;16(7):1832-1844. doi: 10.1016/j.stemcr.2021.05.013. Epub 2021 Jun 17. Stem Cell Reports. 2021. PMID: 34143973 Free PMC article.
-
Sertoli cells dictate spermatogonial stem cell niches in the mouse testis.Biol Reprod. 2011 Apr;84(4):639-45. doi: 10.1095/biolreprod.110.087320. Epub 2010 Nov 17. Biol Reprod. 2011. PMID: 21084712 Free PMC article.
-
Reversible inhibition of the blood-testis barrier protein improves stem cell homing in mouse testes.J Reprod Dev. 2018 Dec 14;64(6):511-522. doi: 10.1262/jrd.2018-093. Epub 2018 Sep 1. J Reprod Dev. 2018. PMID: 30175719 Free PMC article.
-
The spermatogonial stem cell niche.Microsc Res Tech. 2009 Aug;72(8):580-5. doi: 10.1002/jemt.20699. Microsc Res Tech. 2009. PMID: 19263493 Review.
-
The niche for spermatogonial stem cells in the mammalian testis.Int J Hematol. 2005 Dec;82(5):381-8. doi: 10.1532/IJH97.05088. Int J Hematol. 2005. PMID: 16533739 Review.
Cited by
-
Spermatogonial stem cell technologies: applications from human medicine to wildlife conservation†.Biol Reprod. 2024 Oct 14;111(4):757-779. doi: 10.1093/biolre/ioae109. Biol Reprod. 2024. PMID: 38993049 Free PMC article. Review.
-
Establishment of Etv5 gene knockout mice as a recipient model for spermatogonial stem cell transplantation.Biol Open. 2021 Jan 6;10(1):bio056804. doi: 10.1242/bio.056804. Biol Open. 2021. PMID: 33298570 Free PMC article.
-
In quest of genomic treasure.J Reprod Dev. 2015;61(6):489-93. doi: 10.1262/jrd.2015-098. Epub 2015 Sep 20. J Reprod Dev. 2015. PMID: 26400375 Free PMC article.
-
TSPAN8 Expression Distinguishes Spermatogonial Stem Cells in the Prepubertal Mouse Testis.Biol Reprod. 2016 Dec;95(6):117. doi: 10.1095/biolreprod.116.144220. Epub 2016 Oct 12. Biol Reprod. 2016. PMID: 27733379 Free PMC article.
-
Skp1-Cullin-F-box (SCF)-type ubiquitin ligase FBXW7 negatively regulates spermatogonial stem cell self-renewal.Proc Natl Acad Sci U S A. 2014 Jun 17;111(24):8826-31. doi: 10.1073/pnas.1401837111. Epub 2014 May 30. Proc Natl Acad Sci U S A. 2014. PMID: 24879440 Free PMC article.
References
-
- Meng X, Lindahl M, Hyvönen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, Saarma M, Sariola H. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 2000; 287: 1489–1493 - PubMed
-
- Kanatsu-Shinohara M, Toyokuni S, Morimoto T, Matsui S, Honjo T, Shinohara T. Functional assessment of self-renewal activity of male germline stem cells following cytotoxic damage and serial transplantation. Biol Reprod 2003; 68: 1801–1807 - PubMed
-
- Meistrich ML, van Beek MEAB. Spermatogonial stem cells. In: Desjardins CC, Ewing LL (eds.), Cell and Molecular Biology of the Testis. New York: Oxford University Press; 1993: 266–295.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

