Toxicity and outcome of radiotherapy with concomitant and adjuvant temozolomide in elderly patients with glioblastoma: a retrospective study
- PMID: 24257502
- PMCID: PMC4533479
- DOI: 10.2176/nmc.oa2012-0441
Toxicity and outcome of radiotherapy with concomitant and adjuvant temozolomide in elderly patients with glioblastoma: a retrospective study
Abstract
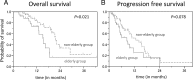
Radiation therapy with concomitant and adjuvant temozolomide (TMZ) is the standard therapy for nonelderly patients with glioblastoma. However, TMZ-based chemoradiotherapy for elderly patients with glioblastoma is controversial. The aim of this study was to investigate the benefits and adverse effects of this combined therapy in elderly patients with glioblastoma. Of the 76 newly diagnosed glioblastoma patients who were treated with standard radiotherapy (60 Gy/30 fractions) and TMZ, treatment toxicity and therapeutic outcome were evaluated in 27 elderly patients (age 65 years or older) and compared with those of 49 nonelderly counterparts (age younger than 65 years). The incidence of common toxicity criteria Grade 4 adverse events during the concomitant course was higher in the elderly group than that in the nonelderly group (26% versus 8%; p = 0.046). Cognitive dysfunction was observed only in the elderly group (p = 0.042). The median overall survival (OS) and median progression-free survival in the elderly group were 15.2 months (95% confidence interval [CI]; 12.9-18.5) and 8.4 months (95% CI; 5.1-11.7), respectively. OS was significantly shorter in the elderly group than in the nonelderly group (p = 0.021). The recursive partitioning analysis score was a prognostic factor for OS. TMZ-based chemoradiotherapy was associated with an increased risk of Grade 4 adverse events in the elderly patients during concomitant use. Thus, elderly patients who undergo a concomitant course of TMZ must be closely monitored for adverse events. Treatment of glioblastoma in elderly patients must be optimized to reduce toxicity to acceptable levels and to maintain efficacy.
Conflict of interest statement
All authors have no conflict of interest to disclose.
Figures
References
-
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. (eds): WHO classification of tumours of the central nervous system. Lyon, International Agency for Research on Cancer, 2007.
-
- Laperriere N, Weller M, Stupp R, Perry JR, Brandes AA, Wick W, van den Bent MJ: Optimal management of elderly patients with glioblastoma. Cancer Treat Rev 39: 350– 357, 2013. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group : Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987– 996, 2005. - PubMed
-
- Laperriere N, O'Callaghan C, Ding K: Rationale and design for a phase III randomized controlled trial in elderly patients with glioblastoma multiforme: NCIC CTG CE. Presented at the 13th Biennial Canadian Neuro-Oncology Meeting, May 16–18, 2008; Banff, Alberta, Canada
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials