Highly pathological influenza A virus infection is associated with augmented expression of PD-1 by functionally compromised virus-specific CD8+ T cells
- PMID: 24257598
- PMCID: PMC3911576
- DOI: 10.1128/JVI.02851-13
Highly pathological influenza A virus infection is associated with augmented expression of PD-1 by functionally compromised virus-specific CD8+ T cells
Abstract
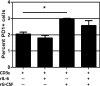
One question that continues to challenge influenza A research is why some strains of virus are so devastating compared to their more mild counterparts. We approached this question from an immunological perspective, investigating the CD8(+) T cell response in a mouse model system comparing high- and low-pathological influenza virus infections. Our findings reveal that the early (day 0 to 5) viral titer was not the determining factor in the outcome of disease. Instead, increased numbers of antigen-specific CD8(+) T cells and elevated effector function on a per-cell basis were found in the low-pathological infection and correlated with reduced illness and later-time-point (day 6 to 10) viral titer. High-pathological infection was associated with increased PD-1 expression on influenza virus-specific CD8(+) T cells, and blockade of PD-L1 in vivo led to reduced virus titers and increased CD8(+) T cell numbers in high- but not low-pathological infection, though T cell functionality was not restored. These data show that high-pathological acute influenza virus infection is associated with a dysregulated CD8(+) T cell response, which is likely caused by the more highly inflamed airway microenvironment during the early days of infection. Therapeutic approaches specifically aimed at modulating innate airway inflammation may therefore promote efficient CD8(+) T cell activity. We show that during a severe influenza virus infection, one type of immune cell, the CD8 T cell, is less abundant and less functional than in a more mild infection. This dysregulated T cell phenotype correlates with a lower rate of virus clearance in the severe infection and is partially regulated by the expression of a suppressive coreceptor called PD-1. Treatment with an antibody that blocks PD-1 improves T cell functionality and increases virus clearance.
Figures








Similar articles
-
Long-term surviving influenza infected cells evade CD8+ T cell mediated clearance.PLoS Pathog. 2019 Sep 26;15(9):e1008077. doi: 10.1371/journal.ppat.1008077. eCollection 2019 Sep. PLoS Pathog. 2019. PMID: 31557273 Free PMC article.
-
Genetic absence of PD-L1 does not restore CD8+ T cell function during respiratory virus infection and delays virus clearance.J Virol. 2024 Oct 22;98(10):e0079724. doi: 10.1128/jvi.00797-24. Epub 2024 Sep 23. J Virol. 2024. PMID: 39311697 Free PMC article.
-
Gamma interferon regulates contraction of the influenza virus-specific CD8 T cell response and limits the size of the memory population.J Virol. 2013 Dec;87(23):12510-22. doi: 10.1128/JVI.01776-13. Epub 2013 Sep 11. J Virol. 2013. PMID: 24027334 Free PMC article.
-
Human influenza viruses and CD8(+) T cell responses.Curr Opin Virol. 2016 Feb;16:132-142. doi: 10.1016/j.coviro.2016.01.016. Epub 2016 Mar 12. Curr Opin Virol. 2016. PMID: 26974887 Review.
-
CD8 T cell responses to influenza virus infection in aged mice.Ageing Res Rev. 2011 Sep;10(4):422-7. doi: 10.1016/j.arr.2011.02.001. Epub 2011 Feb 15. Ageing Res Rev. 2011. PMID: 21315186 Free PMC article. Review.
Cited by
-
Activation of TREK-1 (K2P2.1) potassium channels protects against influenza A-induced lung injury.Am J Physiol Lung Cell Mol Physiol. 2023 Jan 1;324(1):L64-L75. doi: 10.1152/ajplung.00116.2022. Epub 2022 Nov 21. Am J Physiol Lung Cell Mol Physiol. 2023. PMID: 36410022 Free PMC article.
-
Epidemiology and Immune Pathogenesis of Viral Sepsis.Front Immunol. 2018 Sep 27;9:2147. doi: 10.3389/fimmu.2018.02147. eCollection 2018. Front Immunol. 2018. PMID: 30319615 Free PMC article. Review.
-
Selective dependence on IL-7 for antigen-specific CD8 T cell responses during airway influenza infection.Sci Rep. 2022 Jan 7;12(1):135. doi: 10.1038/s41598-021-03936-y. Sci Rep. 2022. PMID: 34997007 Free PMC article.
-
Considerations of CD8+ T Cells for Optimized Vaccine Strategies Against Respiratory Viruses.Front Immunol. 2022 Jun 15;13:918611. doi: 10.3389/fimmu.2022.918611. eCollection 2022. Front Immunol. 2022. PMID: 35774782 Free PMC article. Review.
-
Bioinformatic analysis of PD-1 checkpoint blockade response in influenza infection.BMC Genom Data. 2022 Aug 13;23(1):65. doi: 10.1186/s12863-022-01081-7. BMC Genom Data. 2022. PMID: 35962325 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

