Assessing the susceptibility of transgenic mice overexpressing deer prion protein to bovine spongiform encephalopathy
- PMID: 24257620
- PMCID: PMC3911610
- DOI: 10.1128/JVI.02762-13
Assessing the susceptibility of transgenic mice overexpressing deer prion protein to bovine spongiform encephalopathy
Abstract
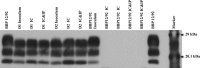
Several transgenic mouse models have been developed which facilitate the transmission of chronic wasting disease (CWD) of cervids and allow prion strain discrimination. The present study was designed to assess the susceptibility of the prototypic mouse line, Tg(CerPrP)1536(+/-), to bovine spongiform encephalopathy (BSE) prions, which have the ability to overcome species barriers. Tg(CerPrP)1536(+/-) mice challenged with red deer-adapted BSE resulted in 90% to 100% attack rates, and BSE from cattle failed to transmit, indicating agent adaptation in the deer.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

