Feasibility and safety of early combined cognitive and physical therapy for critically ill medical and surgical patients: the Activity and Cognitive Therapy in ICU (ACT-ICU) trial
- PMID: 24257969
- PMCID: PMC3943568
- DOI: 10.1007/s00134-013-3136-0
Feasibility and safety of early combined cognitive and physical therapy for critically ill medical and surgical patients: the Activity and Cognitive Therapy in ICU (ACT-ICU) trial
Abstract
Purpose: Cognitive impairment after critical illness is common and debilitating. We developed a cognitive therapy program for critically ill patients and assessed the feasibility and safety of administering combined cognitive and physical therapy early during a critical illness.
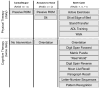
Methods: We randomized 87 medical and surgical ICU patients with respiratory failure and/or shock in a 1:1:2 manner to three groups: usual care, early once-daily physical therapy, or early once-daily physical therapy plus a novel, progressive, twice-daily cognitive therapy protocol. Cognitive therapy included orientation, memory, attention, and problem-solving exercises, and other activities. We assessed feasibility outcomes of the early cognitive plus physical therapy intervention. At 3 months, we also assessed cognitive, functional, and health-related quality of life outcomes. Data are presented as median (interquartile range) or frequency (%).
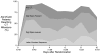
Results: Early cognitive therapy was a delivered to 41/43 (95%) of cognitive plus physical therapy patients on 100% (92-100%) of study days beginning 1.0 (1.0-1.0) day following enrollment. Physical therapy was received by 17/22 (77%) of usual care patients, by 21/22 (95%) of physical therapy only patients, and 42/43 (98%) of cognitive plus physical therapy patients on 17% (10-26%), 67% (46-87%), and 75% (59-88%) of study days, respectively. Cognitive, functional, and health-related quality of life outcomes did not differ between groups at 3-month follow-up.
Conclusions: This pilot study demonstrates that early rehabilitation can be extended beyond physical therapy to include cognitive therapy. Future work to determine optimal patient selection, intensity of treatment, and benefits of cognitive therapy in the critically ill is needed.
Conflict of interest statement
Dr. Ely has received research grants and/or honoraria from Hospira, Orion, and Abbott. Dr. Girard has received honoraria from Hospira. Dr. Pandharipande has received a research grant from Hospira and honoraria from Hospira, and Orion Pharma. Ms. Pun has received honoraria from Hospira. Ms. Boehm has received honoraria from Hospira. Dr. Gill has received honoraria from Novartis. The other authors report no financial disclosures.
Figures

Comment in
-
Early cognitive and physical rehabilitation: one step towards improving post-critical illness outcomes.Intensive Care Med. 2014 Mar;40(3):442-4. doi: 10.1007/s00134-014-3233-8. Epub 2014 Feb 25. Intensive Care Med. 2014. PMID: 24567227 No abstract available.
References
-
- Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF., Jr Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171:340–347. - PubMed
-
- Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, Kudlow P, Cook D, Slutsky AS, Cheung AM. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. - PubMed
Publication types
MeSH terms
Grants and funding
- UL1TR000445/TR/NCATS NIH HHS/United States
- R03AG045095/AG/NIA NIH HHS/United States
- P30 AG021342/AG/NIA NIH HHS/United States
- R01 AG035117/AG/NIA NIH HHS/United States
- R01AG035117/AG/NIA NIH HHS/United States
- P30AG021342/AG/NIA NIH HHS/United States
- R01AG027472/AG/NIA NIH HHS/United States
- K24AG021507/AG/NIA NIH HHS/United States
- R01HL111111/HL/NHLBI NIH HHS/United States
- R03 AG045095/AG/NIA NIH HHS/United States
- K07AG043587/AG/NIA NIH HHS/United States
- K07 AG043587/AG/NIA NIH HHS/United States
- UL1 TR000445/TR/NCATS NIH HHS/United States
- T32HL087738/HL/NHLBI NIH HHS/United States
- R01 HL111111/HL/NHLBI NIH HHS/United States
- K23 AG031322/AG/NIA NIH HHS/United States
- K23 AG034257/AG/NIA NIH HHS/United States
- K23AG031322/AG/NIA NIH HHS/United States
- K23AG034257/AG/NIA NIH HHS/United States
- T32 HL087738/HL/NHLBI NIH HHS/United States
- R01 AG027472/AG/NIA NIH HHS/United States
- K24 AG021507/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

