Accumulation of hydroxyethyl starch in human and animal tissues: a systematic review
- PMID: 24257970
- PMCID: PMC7728635
- DOI: 10.1007/s00134-013-3156-9
Accumulation of hydroxyethyl starch in human and animal tissues: a systematic review
Erratum in
-
Correction to: Accumulation of hydroxyethyl starch in human and animal tissues: a systematic review.Intensive Care Med. 2021 Feb;47(2):263. doi: 10.1007/s00134-020-06269-y. Intensive Care Med. 2021. PMID: 33052420 Free PMC article. No abstract available.
Abstract
Purpose: To systematically review clinical and preclinical data on hydroxyethyl starch (HES) tissue storage.
Methods: MEDLINE (PubMed) was searched and abstracts were screened using defined criteria to identify articles containing original data on HES tissue accumulation.
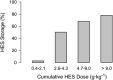
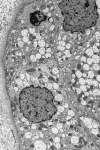
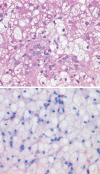
Results: Forty-eight studies were included: 37 human studies with a total of 635 patients and 11 animal studies. The most frequent indication for fluid infusion was surgery accounting for 282 patients (45.9%). HES localization in skin was shown by 17 studies, in kidney by 12, in liver by 8, and in bone marrow by 5. Additional sites of HES deposition were lymph nodes, spleen, lung, pancreas, intestine, muscle, trophoblast, and placental stroma. Among major organs the highest measured tissue concentration of HES was in the kidney. HES uptake into intracellular vacuoles was observed by 30 min after infusion. Storage was cumulative, increasing in proportion to dose, although in 15% of patients storage and associated symptoms were demonstrated at the lowest cumulative doses (0.4 g kg(-1)). Some HES deposits were extremely long-lasting, persisting for 8 years or more in skin and 10 years in kidney. Pruritus associated with HES storage was described in 17 studies and renal dysfunction in ten studies. In one included randomized trial, HES infusion produced osmotic nephrosis-like lesions indicative of HES storage (p = 0.01) and also increased the need for renal replacement therapy (odds ratio, 9.50; 95% confidence interval, 1.09-82.7; p = 0.02). The tissue distribution of HES was generally similar in animals and humans.
Conclusions: Tissue storage of HES is widespread, rapid, cumulative, frequently long-lasting, and potentially harmful.
Figures
Comment in
-
What's new in the controversy on the renal/tissue toxicity of starch solutions?Intensive Care Med. 2014 Mar;40(3):427-30. doi: 10.1007/s00134-013-3191-6. Epub 2014 Jan 17. Intensive Care Med. 2014. PMID: 24435202 No abstract available.
References
-
- Thompson WL, Fukushima T, Rutherford RB, Walton RP. Intravascular persistence, tissue storage, and excretion of hydroxyethyl starch. Surg Gynecol Obstet. 1970;131:965–972. - PubMed
-
- Lindblad G, Falk J. Konzentrationsverlauf von Hydroxyäthylstärke und Dextran in Serum und Lebergewebe von Kaninchen und die histopathologischen Folgen der Speicherung von Hydroxyäthylstärke. Infusionstherapie. 1976;3:301–303. - PubMed
-
- Jesch F, Hübner G, Zumtobel V, Zimmermann M, Messmer K. Hydroxyäthylstärke (HÄS 450/0,7) in Plasma und Leber: Konzentrationsverlauf und histologische Veränderungen beim Menschen. Infusionsther Klin Ernahr. 1979;6:112–117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

