Lipid droplet autophagy in the yeast Saccharomyces cerevisiae
- PMID: 24258026
- PMCID: PMC3890349
- DOI: 10.1091/mbc.E13-08-0448
Lipid droplet autophagy in the yeast Saccharomyces cerevisiae
Abstract
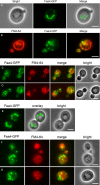
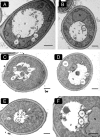
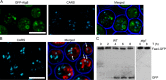
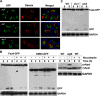
Cytosolic lipid droplets (LDs) are ubiquitous organelles in prokaryotes and eukaryotes that play a key role in cellular and organismal lipid homeostasis. Triacylglycerols (TAGs) and steryl esters, which are stored in LDs, are typically mobilized in growing cells or upon hormonal stimulation by LD-associated lipases and steryl ester hydrolases. Here we show that in the yeast Saccharomyces cerevisiae, LDs can also be turned over in vacuoles/lysosomes by a process that morphologically resembles microautophagy. A distinct set of proteins involved in LD autophagy is identified, which includes the core autophagic machinery but not Atg11 or Atg20. Thus LD autophagy is distinct from endoplasmic reticulum-autophagy, pexophagy, or mitophagy, despite the close association between these organelles. Atg15 is responsible for TAG breakdown in vacuoles and is required to support growth when de novo fatty acid synthesis is compromised. Furthermore, none of the core autophagy proteins, including Atg1 and Atg8, is required for LD formation in yeast.
Figures

Similar articles
-
Pex3-anchored Atg36 tags peroxisomes for degradation in Saccharomyces cerevisiae.EMBO J. 2012 Jun 29;31(13):2852-68. doi: 10.1038/emboj.2012.151. Epub 2012 May 29. EMBO J. 2012. PMID: 22643220 Free PMC article.
-
Pexophagy in yeasts.Biochim Biophys Acta. 2016 May;1863(5):992-8. doi: 10.1016/j.bbamcr.2015.09.023. Epub 2015 Sep 26. Biochim Biophys Acta. 2016. PMID: 26409485 Review.
-
The autophagy-related protein kinase Atg1 interacts with the ubiquitin-like protein Atg8 via the Atg8 family interacting motif to facilitate autophagosome formation.J Biol Chem. 2012 Aug 17;287(34):28503-7. doi: 10.1074/jbc.C112.387514. Epub 2012 Jul 9. J Biol Chem. 2012. PMID: 22778255 Free PMC article.
-
Phosphorylation of mitophagy and pexophagy receptors coordinates their interaction with Atg8 and Atg11.EMBO Rep. 2013 May;14(5):441-9. doi: 10.1038/embor.2013.40. Epub 2013 Apr 5. EMBO Rep. 2013. PMID: 23559066 Free PMC article.
-
Lipid droplet dynamics in budding yeast.Cell Mol Life Sci. 2015 Jul;72(14):2677-95. doi: 10.1007/s00018-015-1903-5. Epub 2015 Apr 18. Cell Mol Life Sci. 2015. PMID: 25894691 Free PMC article. Review.
Cited by
-
Coherent Raman scattering microscopy of lipid droplets in cells and tissues.J Raman Spectrosc. 2023 Sep;54(9):988-1000. doi: 10.1002/jrs.6540. Epub 2023 May 7. J Raman Spectrosc. 2023. PMID: 38076450 Free PMC article.
-
Membrane dynamics and protein targets of lipid droplet microautophagy during ER stress-induced proteostasis in the budding yeast, Saccharomyces cerevisiae.Autophagy. 2021 Sep;17(9):2363-2383. doi: 10.1080/15548627.2020.1826691. Epub 2020 Oct 6. Autophagy. 2021. PMID: 33021864 Free PMC article.
-
Topology and Function of the S. cerevisiae Autophagy Protein Atg15.Cells. 2023 Aug 12;12(16):2056. doi: 10.3390/cells12162056. Cells. 2023. PMID: 37626866 Free PMC article.
-
Neutral lipid stores and lipase PNPLA5 contribute to autophagosome biogenesis.Curr Biol. 2014 Mar 17;24(6):609-20. doi: 10.1016/j.cub.2014.02.008. Epub 2014 Mar 6. Curr Biol. 2014. PMID: 24613307 Free PMC article.
-
The assembly of lipid droplets and their roles in challenged cells.EMBO J. 2018 Jun 15;37(12):e98947. doi: 10.15252/embj.201898947. Epub 2018 May 22. EMBO J. 2018. PMID: 29789390 Free PMC article. Review.
References
-
- Athenstaedt K, Daum G. Tgl4p and Tgl5p, two triacylglycerol lipases of the yeast Saccharomyces cerevisiae are localized to lipid particles. J Biol Chem. 2005;280:37301–37309. - PubMed
-
- Cheong H, Klionsky DJ. Biochemical methods to monitor autophagy-related processes in yeast. Methods Enzymol. 2008;451:1–426. - PubMed
-
- Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–725. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

