Mechanosensitive channels and bacterial cell wall integrity: does life end with a bang or a whimper?
- PMID: 24258154
- PMCID: PMC3869158
- DOI: 10.1098/rsif.2013.0850
Mechanosensitive channels and bacterial cell wall integrity: does life end with a bang or a whimper?
Abstract
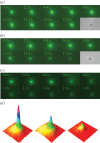
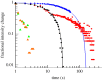
Mechanogated channels are fundamental components of bacterial cells that enable retention of physical integrity during extreme increases in cell turgor. Optical tweezers combined with microfluidics have been used to study the fate of individual Escherichia coli cells lacking such channels when subjected to a bursting stress caused by increased turgor. Fluorescence-activated cell sorting and electron microscopy complement these studies. These analyses show that lysis occurs with a high probability, but the precise path differs between individual cells. By monitoring the loss of cytoplasmic green fluorescent protein, we have determined that some cells release this protein but remain phase dark (granular) consistent with the retention of the majority of large proteins. By contrast, most cells suffer cataclysmic wall failure leading to loss of granularity but with the retention of DNA and overall cell shape (protein-depleted ghosts). The time span of these events induced by hypo-osmotic shock varies but is of the order of milliseconds. The data are interpreted in terms of the timing of mechanosensitive channel gating relative to osmotically induced water influx.
Keywords: (bacterial) cell wall; bacterial stress response; fluorescence-activated cell sorting; mechanosensitive channels; microfluidics; optical tweezers.
Figures


References
-
- Mitchell P, Moyle J. 1956. Osmotic function and structure in bacteria. In Bacterial anatomy (eds Spooner E, Stocker B.), pp. 150–180. Cambridge, UK: Cambridge University Press.
-
- Booth IR, Cairney J, Sutherland L, Higgin CF. 1988. Enteric bacteria and osmotic stress: an integrated homeostatic system. Soc. Appl. Bacteriol. Symp. Ser. 17, 35S–49S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

