RNA-binding protein GLD-1/quaking genetically interacts with the mir-35 and the let-7 miRNA pathways in Caenorhabditis elegans
- PMID: 24258276
- PMCID: PMC3843822
- DOI: 10.1098/rsob.130151
RNA-binding protein GLD-1/quaking genetically interacts with the mir-35 and the let-7 miRNA pathways in Caenorhabditis elegans
Abstract
Messenger RNA translation is regulated by RNA-binding proteins and small non-coding RNAs called microRNAs. Even though we know the majority of RNA-binding proteins and microRNAs that regulate messenger RNA expression, evidence of interactions between the two remain elusive. The role of the RNA-binding protein GLD-1 as a translational repressor is well studied during Caenorhabditis elegans germline development and maintenance. Possible functions of GLD-1 during somatic development and the mechanism of how GLD-1 acts as a translational repressor are not known. Its human homologue, quaking (QKI), is essential for embryonic development. Here, we report that the RNA-binding protein GLD-1 in C. elegans affects multiple microRNA pathways and interacts with proteins required for microRNA function. Using genome-wide RNAi screening, we found that nhl-2 and vig-1, two known modulators of miRNA function, genetically interact with GLD-1. gld-1 mutations enhance multiple phenotypes conferred by mir-35 and let-7 family mutants during somatic development. We used stable isotope labelling with amino acids in cell culture to globally analyse the changes in the proteome conferred by let-7 and gld-1 during animal development. We identified the histone mRNA-binding protein CDL-1 to be, in part, responsible for the phenotypes observed in let-7 and gld-1 mutants. The link between GLD-1 and miRNA-mediated gene regulation is further supported by its biochemical interaction with ALG-1, CGH-1 and PAB-1, proteins implicated in miRNA regulation. Overall, we have uncovered genetic and biochemical interactions between GLD-1 and miRNA pathways.
Keywords: Caenorhabditis elegans; SILAC; gld-1; let-7; miRNA.
Figures






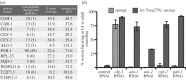
References
-
- Selbach M, Schwanhäusser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. 2008. Widespread changes in protein synthesis induced by microRNAs. Nature 455, 58–63 (doi:10.1038/nature07228) - DOI - PubMed
-
- Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. 2008. The impact of microRNAs on protein output. Nature 455, 64–71 (doi:10.1038/nature07242) - DOI - PMC - PubMed
-
- Krol J, Loedige I, Filipowicz W. 2010. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 11, 597–610 (doi:10.1038/nrg2843) - DOI - PubMed
-
- Caudy AA, et al. 2003. A micrococcal nuclease homologue in RNAi effector complexes. Nature 425, 411–414 (doi:10.1038/nature01956) - DOI - PubMed
-
- Hammell CM, Lubin I, Boag PR, Blackwell TK, Ambros V. 2009. nhl-2 modulates microRNA activity in Caenorhabditis elegans. Cell 136, 926–938 (doi:10.1016/j.cell.2009.01.053) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
