A phase 2 study of ruxolitinib, an oral JAK1 and JAK2 Inhibitor, in patients with advanced polycythemia vera who are refractory or intolerant to hydroxyurea
- PMID: 24258498
- PMCID: PMC4231215
- DOI: 10.1002/cncr.28441
A phase 2 study of ruxolitinib, an oral JAK1 and JAK2 Inhibitor, in patients with advanced polycythemia vera who are refractory or intolerant to hydroxyurea
Abstract
Background: Polycythemia vera (PV) is a myeloproliferative neoplasm associated with somatic gain-of-function mutations of Janus kinase-2 (JAK2). Therapeutic options are limited in patients with advanced disease. Ruxolitinib, an oral JAK1/JAK2 inhibitor, is active in preclinical models of PV. The long-term efficacy and safety of ruxolitinib in patients with advanced PV who are refractory or intolerant to hydroxyurea were studied in a phase 2 trial.
Methods: Response was assessed using modified European LeukemiaNet criteria, which included a reduction in hematocrit to <45% without phlebotomy, resolution of palpable splenomegaly, normalization of white blood cell and platelet counts, and reduction in PV-associated symptoms.
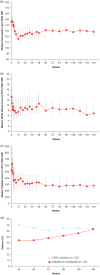
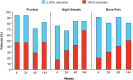
Results: Thirty-four patients received ruxolitinib for a median of 152 weeks (range, 31 weeks-177 weeks) or 35.0 months (range, 7.1 months-40.7 months). Hematocrit <45% without phlebotomy was achieved in 97% of patients by week 24.Only 1 patient required a phlebotomy after week 4. Among patients with palpable splenomegaly at baseline, 44% and 63%, respectively, achieved nonpalpable spleen measurements at weeks 24 and 144. Clinically meaningful improvements in pruritus, night sweats, and bone pain were observed within 4 weeks of the initiation of therapy and maintained with continued treatment. Ruxolitinib treatment also reduced elevated levels of inflammatory cytokines and granulocyte activation. Thrombocytopenia and anemia were the most common adverse events.Thrombocytopenia of grade 3 or anemia of grade 3 (according to National Cancer Institute Common Terminology Criteria for Adverse Events,version 3.0) occurred in 3 patients each (9%) (1 patient had both) and were managed with dose modification.
Conclusions: Ruxolitinib was generally well tolerated and provided rapid and durable clinical benefits in patients with advanced PV who were refractory or intolerant to hydroxyurea.
Figures

References
-
- Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med. 2004;117:755–761. - PubMed
-
- Tefferi A. Polycythemia vera and essential thrombocythemia: 2012 update on diagnosis, risk stratification, and management. Am J Hematol. 2012;87:285–293. - PubMed
-
- Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118:401–408. - PubMed
-
- Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-alpha. Cancer. 2006;107:451–458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

