Peptide-induced immune regulation by a promiscuous and immunodominant CD4T-cell epitope of Timothy grass pollen: a role of Cbl-b and Itch in regulation
- PMID: 24258832
- PMCID: PMC4368902
- DOI: 10.1136/thoraxjnl-2013-204324
Peptide-induced immune regulation by a promiscuous and immunodominant CD4T-cell epitope of Timothy grass pollen: a role of Cbl-b and Itch in regulation
Abstract
Background: T-cell targeted peptide epitope tolerogens from grass pollen allergens may be useful in treating seasonal allergic rhinitis, but there is urgent need for optimisation of approaches from improved understanding of mechanism.
Objective: We sought to identify human leukocyte antigen (HLA)-DR1-restricted epitopes from the Timothy grass pollen allergen, Phleum pratense, and characterise T-cell immune regulation following intranasal administration of a single, immunodominant epitope.
Methods: T-cell epitopes within P pratense were identified using HLA-DR1 transgenic mice and tetramer-guided epitope mapping (TGEM) in HLA-DR1-positive individuals with grass allergy. An immunodominant epitope was tested in HLA-DR1 transgenics for impact on responses to whole Phl p5 b or peptide. Microarrays and quantitative PCR were used to characterise T-cell immunity.
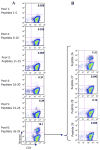
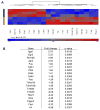
Results: Peptide 26 (p26) was identified in HLA-DR1 transgenic mice and by TGEM analysis of HLA-DR1-positive individuals with grass allergy. p26 shows promiscuous binding to a wide range of HLA class II alleles, making it of relevance across immunogenetically diverse patients. The epitope is conserved in rye and velvet grass, making it applicable across a spectrum of grass pollen allergy. Intranasal pretreatment of mice with p26 results in significantly reduced T-cell responses. Transcriptomic array analysis in mice showed T-cell regulation in the intranasal treatment group associated with increased expression of members of the Cbl-b and Itch E3 ubiquitin ligase pathway.
Conclusions: We defined an immunodominant P pratense epitope, p26, with broad binding across multiple HLA class II alleles. Intranasal treatment of mice with p26 results in T-cell regulation to whole allergen, involving the Cbl-b and Itch regulatory pathway.
Keywords: Allergic lung disease; Lymphocyte Biology.
Conflict of interest statement
Figures


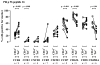


References
-
- Meltzer EO. The prevalence and medical and economic impact of allergic rhinitis in the United States. J Allergy Clin Immunol. 1997;99(6 Pt 2):S805–28. - PubMed
-
- Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–75. - PubMed
-
- Durham SR, Emminger W, Kapp A, et al. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129:717–25. e5. - PubMed
-
- Walker SM, Durham SR, Till SJ, et al. Immunotherapy for allergic rhinitis. Clin Exp Allergy. 2011;41:1177–200. - PubMed
-
- Oldfield WL, Larche M, Kay AB. Effect of T-cell peptides derived from Fel d 1 on allergic reactions and cytokine production in patients sensitive to cats: a randomised controlled trial. Lancet. 2002;360:47–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
