Stem cell-derived endochondral cartilage stimulates bone healing by tissue transformation
- PMID: 24259230
- PMCID: PMC4802866
- DOI: 10.1002/jbmr.2148
Stem cell-derived endochondral cartilage stimulates bone healing by tissue transformation
Abstract
Although bone has great capacity for repair, there are a number of clinical situations (fracture non-unions, spinal fusions, revision arthroplasty, segmental defects) in which auto- or allografts attempt to augment bone regeneration by promoting osteogenesis. Critical failures associated with current grafting therapies include osteonecrosis and limited integration between graft and host tissue. We speculated that the underlying problem with current bone grafting techniques is that they promote bone regeneration through direct osteogenesis. Here we hypothesized that using cartilage to promote endochondral bone regeneration would leverage normal developmental and repair sequences to produce a well-vascularized regenerate that integrates with the host tissue. In this study, we use a translational murine model of a segmental tibia defect to test the clinical utility of bone regeneration from a cartilage graft. We further test the mechanism by which cartilage promotes bone regeneration using in vivo lineage tracing and in vitro culture experiments. Our data show that cartilage grafts support regeneration of a vascularized and integrated bone tissue in vivo, and subsequently propose a translational tissue engineering platform using chondrogenesis of mesenchymal stem cells (MSCs). Interestingly, lineage tracing experiments show the regenerate was graft derived, suggesting transformation of the chondrocytes into bone. In vitro culture data show that cartilage explants mineralize with the addition of bone morphogenetic protein (BMP) or by exposure to human vascular endothelial cell (HUVEC)-conditioned medium, indicating that endothelial cells directly promote ossification. This study provides preclinical data for endochondral bone repair that has potential to significantly improve patient outcomes in a variety of musculoskeletal diseases and injuries. Further, in contrast to the dogmatic view that hypertrophic chondrocytes undergo apoptosis before bone formation, our data suggest cartilage can transform into bone by activating the pluripotent transcription factor Oct4A. Together these data represent a paradigm shift describing the mechanism of endochondral bone repair and open the door for novel regenerative strategies based on improved biology.
Keywords: BIOENGINEERING; CARTILAGE BIOLOGY; CHONDROCYTES; INJURY/FRACTURE HEALING; MOLECULAR PATHWAYS; REMODELING; THERAPEUTICS.
© 2014 American Society for Bone and Mineral Research.
Conflict of interest statement
There are no competing or conflicts of interest to report related to the work presented in this manuscript for any of the authors. Author contribution to the paper was as follows: study design: CSB, DPH, TM, RSM; study conduct and data collection: CSB, DPH, AJT, FF; Data interpretation: CSB, DPH, AJT, FF, BJ, TM, RSM; Drafting manuscript: CSB; Revising Manuscript: CSB, RM; Approval of final version of manuscript: CSB, DPH, AJT, FF, BJ, TM, RSM. CSB take responsibility for the integrity of the data.
Figures





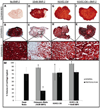

References
-
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–2260. Epub 2012/12/19. doi: 10.1016/S0140-6736(12)61766-8 S0140-6736(12)61766-8 [pii]. PubMed PMID: 23245609. - PMC - PubMed
-
- Agency for Healthcare Research and Quality. Available from: http://www.ahrq.gov/news/nn/nn012506.htm. - PubMed
-
- The Burden of Musculoskeletal Diseases in the United States. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
-
- Brigman BE, Hornicek FJ, Gebhardt MC, Mankin HJ. Allografts about the Knee in Young Patients with High-Grade Sarcoma. Clinical orthopaedics and related research. 2004;(421):232–239. Epub 2004/05/05. PubMed PMID: 15123953. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

