ALX/FPR2 receptor for RvD1 is expressed and functional in salivary glands
- PMID: 24259417
- PMCID: PMC3919993
- DOI: 10.1152/ajpcell.00284.2013
ALX/FPR2 receptor for RvD1 is expressed and functional in salivary glands
Abstract
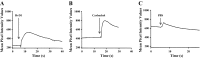
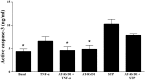
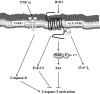
Sjögren's syndrome (SS) is an autoimmune disorder characterized by chronic inflammation and destruction of salivary and lacrimal glands, leading to dry mouth, dry eyes, and the presence of anti-nuclear antibodies. Despite modern advances, the current therapies for SS have no permanent benefit. A potential treatment could involve the use of resolvins, which are highly potent endogenous lipid mediators that are synthesized during the resolution of inflammation to restore tissue homeostasis. Our previous studies indicate that ALX/FPR2, the receptor for RvD1, is expressed and active in the rat parotid cell line Par-C10. Specifically, activation of ALX/FPR2 with RvD1 blocked inflammatory signals caused by TNF-α and enhanced salivary epithelial integrity. The goal of this study was to investigate RvD1 receptor expression and signaling pathways in primary salivary cells. Additionally, we determined the role of the aspirin-triggered 17R analog (AT-RvD1, a more chemically stable RvD1 epimeric form) in prevention of TNF-α-mediated salivary inflammation in mouse submandibular glands (mSMG). Our results indicate that ALX/FPR2 is expressed in mSMG and is able to elicit intracellular Ca2+ responses and phosphorylation of Erk1/2, as well as Akt. Given that these signaling pathways are linked to cell survival, we investigated whether AT-RvD1 was able to prevent programmed cell death in mSMG. Specifically, we determined that AT-RvD1 prevented TNF-α-mediated caspase-3 activation. Finally, we show that ALX/FPR2 is expressed in human minor salivary glands with and without SS, indicating the potential therapeutic use of AT-RvD1 for this condition.
Keywords: ALX/FPR2; AT-RvD1; GPR32; RvD1; Sjögren's syndrome; resolvins; salivary glands.
Figures



References
-
- Azuma M, Aota K, Tamatani T, Motegi K, Yamashita T, Harada K, Hayashi Y, Sato M. Suppression of tumor necrosis factor-α-induced matrix metalloproteinase 9 production by the introduction of a super-repressor form of inhibitor of nuclear factor κBα complementary DNA into immortalized human salivary gland acinar cells. Prevention of the destruction of the acinar structure in Sjogren's syndrome salivary glands. Arthritis Rheum 43: 1756–1767, 2000 - PubMed
-
- Bento AF, Claudino RF, Dutra RC, Marcon R, Calixto JB. Omega-3 fatty acid-derived mediators 17(R)-hydroxy docosahexaenoic acid, aspirin-triggered resolvin D1 and resolvin D2 prevent experimental colitis in mice. J Immunol 187: 1957–1969, 2011 - PubMed
-
- Daniels TE, Fox PC. Salivary and oral components of Sjogren's syndrome. Rheum Dis Clin North Am 18: 571–589, 1992 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

