Etoposide selectively ablates activated T cells to control the immunoregulatory disorder hemophagocytic lymphohistiocytosis
- PMID: 24259502
- PMCID: PMC4177106
- DOI: 10.4049/jimmunol.1302282
Etoposide selectively ablates activated T cells to control the immunoregulatory disorder hemophagocytic lymphohistiocytosis
Abstract
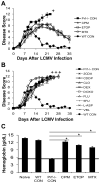
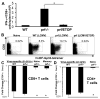
Hemophagocytic lymphohistiocytosis (HLH) is an inborn disorder of immune regulation caused by mutations affecting perforin-dependent cytotoxicity. Defects in this pathway impair negative feedback between cytotoxic lymphocytes and APCs, leading to prolonged and pathologic activation of T cells. Etoposide, a widely used chemotherapeutic drug that inhibits topoisomerase II, is the mainstay of treatment for HLH, although its therapeutic mechanism remains unknown. We used a murine model of HLH, involving lymphocytic choriomeningitis virus infection of perforin-deficient mice, to study the activity and mechanism of etoposide for treating HLH and found that it substantially alleviated all symptoms of murine HLH and allowed prolonged survival. This therapeutic effect was relatively unique among chemotherapeutic agents tested, suggesting distinctive effects on the immune response. We found that the therapeutic mechanism of etoposide in this model system involved potent deletion of activated T cells and efficient suppression of inflammatory cytokine production. This effect was remarkably selective; etoposide did not exert a direct anti-inflammatory effect on macrophages or dendritic cells, and it did not cause deletion of quiescent naive or memory T cells. Finally, etoposide's immunomodulatory effects were similar in wild-type and perforin-deficient animals. Thus, etoposide treats HLH by selectively eliminating pathologic, activated T cells and may have usefulness as a novel immune modulator in a broad array of immunopathologic disorders.
Conflict of interest statement
The authors have no financial conflicts of interest to report.
Figures


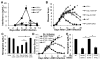

References
-
- Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004;104:735–743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

