Pd-porphyrin-cross-linked implantable hydrogels with oxygen-responsive phosphorescence
- PMID: 24259519
- PMCID: PMC4143977
- DOI: 10.1002/adhm.201300483
Pd-porphyrin-cross-linked implantable hydrogels with oxygen-responsive phosphorescence
Abstract
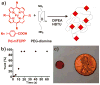
Development of long-term implantable luminescent biosensors for subcutaneous oxygen has proved challenging due to difficulties in immobilizing a biocompatible matrix that prevents sensor aggregation yet maintains sufficient concentration for transdermal optical detection. Here, Pd-porphyrins can be used as PEG cross-linkers to generate a polyamide hydrogel with extreme porphyrin density (≈5 × 10(-3) m). Dye aggregation is avoided due to the spatially constraining 3D mesh formed by the porphyrins themselves. The hydrogel exhibits oxygen-responsive phosphorescence and can be stably implanted subcutaneously in mice for weeks without degradation, bleaching, or host rejection. To further facilitate oxygen detection using steady-state techniques, an oxygen-non-responsive companion hydrogel is developed by blending copper and free base porphyrins to yield intensity-matched luminescence for ratiometric detection.
Keywords: hydrogels; imaging; implants; oxygen sensing; phosphorescence; porphyrins.
© 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



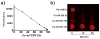

Similar articles
-
Porphyrin-cross-linked hydrogel for fluorescence-guided monitoring and surgical resection.Biomacromolecules. 2011 Sep 12;12(9):3115-8. doi: 10.1021/bm200784s. Epub 2011 Jul 26. Biomacromolecules. 2011. PMID: 21777008
-
Study of the pO2-sensitivity of the dendrimeric and free forms of Pd-meso-tetra(4-carboxyphenyl)porphyrin, incorporated or not in chitosan-based nanoparticles.Chimia (Aarau). 2011;65(9):691-5. doi: 10.2533/chimia.2011.691. Chimia (Aarau). 2011. PMID: 22026181
-
Calibration of Pd-porphyrin phosphorescence for oxygen concentration measurements in vivo.J Appl Physiol (1985). 1996 Nov;81(5):2297-303. doi: 10.1152/jappl.1996.81.5.2297. J Appl Physiol (1985). 1996. PMID: 8941557
-
Hydrogel Based Biosensors for In Vitro Diagnostics of Biochemicals, Proteins, and Genes.Adv Healthc Mater. 2017 Jun;6(12). doi: 10.1002/adhm.201601475. Epub 2017 Mar 31. Adv Healthc Mater. 2017. PMID: 28371450 Review.
-
Measuring oxygen using oxygen dependent quenching of phosphorescence: a status report.Adv Exp Med Biol. 1993;333:225-32. doi: 10.1007/978-1-4899-2468-1_20. Adv Exp Med Biol. 1993. PMID: 8362663 Review. No abstract available.
Cited by
-
Surfactant-Stripped Frozen Pheophytin Micelles for Multimodal Gut Imaging.Adv Mater. 2016 Oct;28(38):8524-8530. doi: 10.1002/adma.201602373. Epub 2016 Jul 11. Adv Mater. 2016. PMID: 27396479 Free PMC article.
-
Fluorescence Spectroscopy of Porphyrins and Phthalocyanines: Some Insights into Supramolecular Self-Assembly, Microencapsulation, and Imaging Microscopy.Molecules. 2021 Jul 14;26(14):4264. doi: 10.3390/molecules26144264. Molecules. 2021. PMID: 34299539 Free PMC article. Review.
-
Color-Tunable Room-Temperature Phosphorescence from Non-Aromatic-Polymer-Involved Charge Transfer.Adv Sci (Weinh). 2024 Aug;11(30):e2404698. doi: 10.1002/advs.202404698. Epub 2024 Jun 14. Adv Sci (Weinh). 2024. PMID: 38874342 Free PMC article.
-
Advanced biomedical hydrogels: molecular architecture and its impact on medical applications.Regen Biomater. 2021 Nov 9;8(6):rbab060. doi: 10.1093/rb/rbab060. eCollection 2021 Dec. Regen Biomater. 2021. PMID: 34925879 Free PMC article. Review.
-
Implantable Tin Porphyrin-PEG Hydrogels with pH-Responsive Fluorescence.Biomacromolecules. 2017 Feb 13;18(2):562-567. doi: 10.1021/acs.biomac.6b01715. Epub 2017 Feb 1. Biomacromolecules. 2017. PMID: 28146351 Free PMC article.
References
-
- Schmedtje JF, Jr, Ji Y-S. Trends Cardiovasc Med. 1998;8:24. - PubMed
-
- López-Lázaro M. Anticancer Agents Med Chem. 2009;9:517. - PubMed
-
- Schreml S, Szeimies Rm, Prantl L, Karrer S, Landthaler M, Babilas P. Br J Dermatol. 2010;163:257. - PubMed
-
- Springett R, Swartz HM. Antioxidants Redox Signal. 2007;9:1295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

