LXRα fuels fatty acid-stimulated oxygen consumption in white adipocytes
- PMID: 24259533
- PMCID: PMC3886663
- DOI: 10.1194/jlr.M043422
LXRα fuels fatty acid-stimulated oxygen consumption in white adipocytes
Abstract
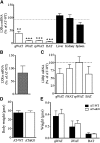
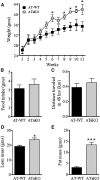
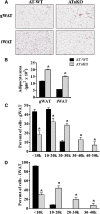
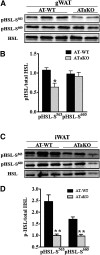
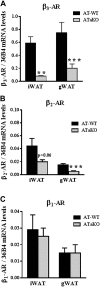
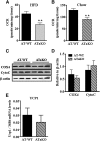
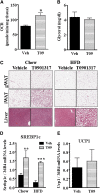
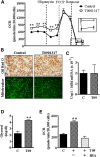
Liver X receptors (LXRs) are transcription factors known for their role in hepatic cholesterol and lipid metabolism. Though highly expressed in fat, the role of LXR in this tissue is not well characterized. We generated adipose tissue LXRα knockout (ATaKO) mice and showed that these mice gain more weight and fat mass on a high-fat diet compared with wild-type controls. White adipose tissue (WAT) accretion in ATaKO mice results from both a decrease in WAT lipolytic and oxidative capacities. This was demonstrated by decreased expression of the β2- and β3-adrenergic receptors, reduced level of phosphorylated hormone-sensitive lipase, and lower oxygen consumption rates (OCRs) in WAT of ATaKO mice. Furthermore, LXR activation in vivo and in vitro led to decreased adipocyte size in WAT and increased glycerol release from primary adipocytes, respectively, with a concomitant increase in OCR in both models. Our findings show that absence of LXRα in adipose tissue results in elevated adiposity through a decrease in WAT oxidation, secondary to attenuated FA availability.
Keywords: adipose tissue; lipolysis; mitochondria; oxidation.
Figures
References
-
- Yach D., Stuckler D., Brownell K. D. 2006. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat. Med. 12: 62–66 - PubMed
-
- Shoelson S. E., Herrero L., Naaz A. 2007. Obesity, inflammation, and insulin resistance. Gastroenterology. 132: 2169–2180 - PubMed
-
- Pi-Sunyer F. X. 1993. Medical hazards of obesity. Ann. Intern. Med. 119: 655–660 - PubMed
-
- Strawford A., Antelo F., Christiansen M., Hellerstein M. K. 2004. Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H2O. Am. J. Physiol. Endocrinol. Metab. 286: E577–E588 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

