Salivary peptide tyrosine-tyrosine 3-36 modulates ingestive behavior without inducing taste aversion
- PMID: 24259562
- PMCID: PMC3834047
- DOI: 10.1523/JNEUROSCI.1064-13.2013
Salivary peptide tyrosine-tyrosine 3-36 modulates ingestive behavior without inducing taste aversion
Abstract
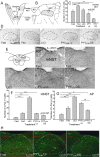
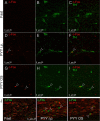
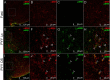
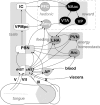
Hormone peptide tyrosine-tyrosine (PYY) is secreted into circulation from the gut L-endocrine cells in response to food intake, thus inducing satiation during interaction with its preferred receptor, Y2R. Clinical applications of systemically administered PYY for the purpose of reducing body weight were compromised as a result of the common side effect of visceral sickness. We describe here a novel approach of elevating PYY in saliva in mice, which, although reliably inducing strong anorexic responses, does not cause aversive reactions. The augmentation of salivary PYY activated forebrain areas known to mediate feeding, hunger, and satiation while minimally affecting brainstem chemoreceptor zones triggering nausea. By comparing neuronal pathways activated by systemic versus salivary PYY, we identified a metabolic circuit associated with Y2R-positive cells in the oral cavity and extending through brainstem nuclei into hypothalamic satiety centers. The discovery of this alternative circuit that regulates ingestive behavior without inducing taste aversion may open the possibility of a therapeutic application of PYY for the treatment of obesity via direct oral application.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
