Selective tuning for contrast in macaque area V4
- PMID: 24259580
- PMCID: PMC6618802
- DOI: 10.1523/JNEUROSCI.3465-13.2013
Selective tuning for contrast in macaque area V4
Erratum in
- J Neurosci. 2014 Feb 5;34(6):2402
Abstract
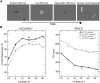
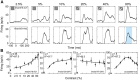
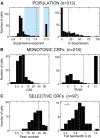
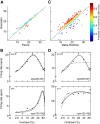
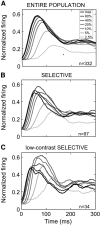
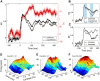
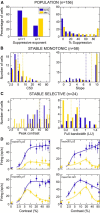
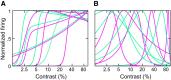
Visually responsive neurons typically exhibit a monotonic-saturating increase of firing with luminance contrast of the stimulus and are able to adapt to the current spatiotemporal context by shifting their selectivity, therefore being perfectly suited for optimal contrast encoding and discrimination. Here we report the first evidence of the existence of neurons showing selective tuning for contrast in area V4d of the behaving macaque (Macaca mulatta), i.e., narrow bandpass filter neurons with peak activity encompassing the whole range of visible contrasts and pronounced attenuation at contrasts higher than the peak. Crucially, we found that contrast tuning emerges after a considerable delay from stimulus onset, likely reflecting the contribution of inhibitory mechanisms. Selective tuning for luminance contrast might support multiple functions, including contrast identification and the attentive selection of low contrast stimuli.
Figures
References
-
- Albrecht DG, Hamilton DB. Striate cortex of monkey and cat: contrast response function. J Neurophysiol. 1982;48:217–237. - PubMed
-
- Albrecht DG, Geisler WS, Frazor RA, Crane AM. Visual cortex neurons of monkeys and cats: temporal dynamics of the contrast response function. J Neurophysiol. 2002;88:888–913. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
