Transfusion-dependent low-risk myelodysplastic patients receiving deferasirox: Long-term follow-up
- PMID: 24260074
- PMCID: PMC3834329
- DOI: 10.3892/ol.2013.1617
Transfusion-dependent low-risk myelodysplastic patients receiving deferasirox: Long-term follow-up
Abstract
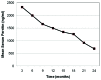
Myelodysplastic syndromes (MDSs) are characterized by ineffective hematopoiesis that results in peripheral cytopenias. Anemia is the most common symptom of MDS and the majority of patients become transfusion-dependent with the risk of iron overload, which may lead to cardiac, hepatic and endocrine complications. Deferasirox is an orally available iron chelator administered once-daily in transfusion-dependent patients with various chronic anemias. Its efficacy has been established in controlled clinical trials. In the present study, we describe our experience with 55 consecutive MDS patients [International Prognostic Scoring System risk score of low (n=32) or intermediate-1 (n=23)] treated with deferasirox in a routine clinical setting following Consensus Guidelines on Iron Chelation Therapy. According to WHO classifications, patients had refractory anemia (n=30), refractory anemia with ringed sideroblasts (n=16), refractory cytopenia with multilineage dysplasia (n=8) or refractory cytopenia with multilineage dysplasia and ringed sideroblasts (n=1). The median monthly transfusion requirement at baseline was 3 units. Patients received a starting dosage of 10 mg/kg/day, subsequently titrated according to serum ferritin (SF) levels which were measured monthly. Safety assessment included monitoring of liver and renal parameters and recording adverse events (AE) during treatment. At the baseline, the mean ± SD SF level was 2,362±172 ng/ml and after 24 months, the mean ± SD decrease in SF was 1,679±209 ng/ml. Sixteen patients had sustained hematological improvement meeting International Working Group 2006 criteria. One patient became transfusion-independent. No severe AE were reported. In conclusion, deferasirox therapy was effective and safe in reducing transfusional iron overload and it reduces transfusion requirement in a subset of patients.
Keywords: chelation; deferasirox; erythroid response; iron overload; myelodysplastic syndromes; transfusion dependence.
Figures
References
-
- Neukirchen J, Fox F, Kündgen A, Nachtkamp K, Strupp C, Haas R, Germing U, Gattermann N. Improved survival in MDS patients receiving iron chelation therapy - a matched pair analysis of 188 patients from the Düsseldorf MDS registry. Leuk Res. 2012;36:1067–1070. - PubMed
-
- Dreyfus F. The deleterious effects of iron overload in patients with myelodysplastic syndromes. Blood Rev. 2008;22(Suppl 2):S29–S34. - PubMed
-
- Malcovati L, Porta MG, Pascutto C, Invernizzi R, Boni M, Travaglino E, Passamonti F, Arcaini L, Maffioli M, Bernasconi P, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23:7594–7603. - PubMed
-
- Delea TE, Hagiwara M, Phatak PD. Retrospective study of the association between transfusion frequency and potential complications of iron overload in patients with myelodysplastic syndrome and other acquired hematopoietic disorders. Curr Med Res Opin. 2009;25:139–147. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous