Paracrine effects of bone marrow-derived endothelial progenitor cells: cyclooxygenase-2/prostacyclin pathway in pulmonary arterial hypertension
- PMID: 24260171
- PMCID: PMC3832480
- DOI: 10.1371/journal.pone.0079215
Paracrine effects of bone marrow-derived endothelial progenitor cells: cyclooxygenase-2/prostacyclin pathway in pulmonary arterial hypertension
Abstract
Background: Endothelial dysfunction is the pathophysiological characteristic of pulmonary arterial hypertension (PAH). Some paracrine factors secreted by bone marrow-derived endothelial progenitor cells (BMEPCs) have the potential to strengthen endothelial integrity and function. This study investigated whether BMEPCs have the therapeutic potential to improve monocrotaline (MCT)-induced PAH via producing vasoprotective substances in a paracrine fashion.
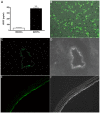
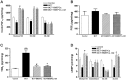
Methods and results: Bone marrow-derived mononuclear cells were cultured for 7 days to yield BMEPCs. 24 hours or 3 weeks after exposure to BMEPCs in vitro or in vivo, the vascular reactivity, cyclooxygenase-2 (COX-2) expression, prostacyclin (PGI2) and cAMP release in isolated pulmonary arteries were examined respectively. Treatment with BMEPCs could improve the relaxation of pulmonary arteries in MCT-induced PAH and BMEPCs were grafted into the pulmonary bed. The COX-2/prostacyclin synthase (PGIS) and its progenies PGI2/cAMP were found to be significantly increased in BMEPCs treated pulmonary arteries, and this action was reversed by a selective COX-2 inhibitor, NS398. Moreover, the same effect was also observed in conditioned medium obtained from BMEPCs culture.
Conclusions: Implantation of BMEPCs effectively ameliorates MCT-induced PAH. Factors secreted in a paracrine fashion from BMEPCs promote vasoprotection by increasing the release of PGI2 and level of cAMP.
Conflict of interest statement
Figures







References
-
- D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, et al. (1991) Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 115: 343–349. - PubMed
-
- Fuster V, Steele PM, Edwards WD, Gersh BJ, McGoon MD, et al. (1984) Primary pulmonary hypertension: natural history and the importance of thrombosis. Circulation 70: 580–587. - PubMed
-
- Christman BW, McPherson CD, Newman JH, King GA, Bernard GR, et al. (1992) An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med 327: 70–75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

