Natural history of progression of HPV infection to cervical lesion or clearance: analysis of the control arm of the large, randomised PATRICIA study
- PMID: 24260180
- PMCID: PMC3834039
- DOI: 10.1371/journal.pone.0079260
Natural history of progression of HPV infection to cervical lesion or clearance: analysis of the control arm of the large, randomised PATRICIA study
Erratum in
- PLoS One. 2013;8(12). doi:10.1371/annotation/cea59317-929c-464a-b3f7-e095248f229a
Abstract
Background: The control arm of PATRICIA (PApilloma TRIal against Cancer In young Adults, NCT00122681) was used to investigate the risk of progression from cervical HPV infection to cervical intraepithelial neoplasia (CIN) or clearance of infection, and associated determinants.
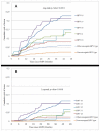
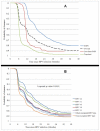
Methods and findings: Women aged 15-25 years were enrolled. A 6-month persistent HPV infection (6MPI) was defined as detection of the same HPV type at two consecutive evaluations over 6 months and clearance as ≥2 type-specific HPV negative samples taken at two consecutive intervals of approximately 6 months following a positive sample. The primary endpoint was CIN grade 2 or greater (CIN2+) associated with the same HPV type as a 6MPI. Secondary endpoints were CIN1+/CIN3+ associated with the same HPV type as a 6MPI; CIN1+/CIN2+/CIN3+ associated with an infection of any duration; and clearance of infection. The analyses included 4825 women with 16,785 infections (3363 women with 6902 6MPIs). Risk of developing a CIN1+/CIN2+/CIN3+ associated with same HPV type as a 6MPI varied with HPV type and was significantly higher for oncogenic versus non-oncogenic types. Hazard ratios for development of CIN2+ were 10.44 (95% CI: 6.96-15.65), 9.65 (5.97-15.60), 5.68 (3.50-9.21), 5.38 (2.87-10.06) and 3.87 (2.38-6.30) for HPV-16, HPV-33, HPV-31, HPV-45 and HPV-18, respectively. HPV-16 or HPV-33 6MPIs had ~25-fold higher risk for progression to CIN3+. Previous or concomitant HPV infection or CIN1+ associated with a different HPV type increased risk. Of the different oncogenic HPV types, HPV-16 and HPV-31 infections were least likely to clear.
Conclusions: Cervical infections with oncogenic HPV types increased the risk of CIN2+ and CIN3+. Previous or concomitant infection or CIN1+ also increased the risk. HPV-16 and HPV-33 have by far the highest risk of progression to CIN3+, and HPV-16 and HPV-31 have the lowest chance of clearance.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

