Coordination between p21 and DDB2 in the cellular response to UV radiation
- PMID: 24260342
- PMCID: PMC3832521
- DOI: 10.1371/journal.pone.0080111
Coordination between p21 and DDB2 in the cellular response to UV radiation
Abstract
The tumor suppressor p53 guides the cellular response to DNA damage mainly by regulating expression of target genes. The cyclin-dependent kinase inhibitor p21, which is induced by p53, can both arrest the cell cycle and inhibit apoptosis. Interestingly, p53-inducible DDB2 (damaged-DNA binding protein 2) promotes apoptosis by mediating p21 degradation after ultraviolet (UV)-induced DNA damage. Here, we developed an integrated model of the p53 network to explore how the UV-irradiated cell makes a decision between survival and death and how the activities of p21 and DDB2 are modulated. By numerical simulations, we found that p53 is activated progressively and the promoter selectivity of p53 depends on its concentration. For minor DNA damage, p53 settles at an intermediate level. p21 is induced by p53 to arrest the cell cycle via inhibiting E2F1 activity, allowing for DNA repair. The proapoptotic genes are expressed at low levels. For severe DNA damage, p53 undergoes a two-phase behavior and accumulates to high levels in the second phase. Consequently, those proapoptotic proteins accumulate remarkably. Bax activates the release of cytochrome c, while DDB2 promotes the degradation of p21, which leads to activation of E2F1 and induction of Apaf-1. Finally, the caspase cascade is activated to trigger apoptosis. We revealed that the downregulation of p21 is necessary for apoptosis induction and PTEN promotes apoptosis by amplifying p53 activation. This work demonstrates that how the dynamics of the p53 network can be finely regulated through feed-forward and feedback loops within the network and emphasizes the importance of p21 regulation in the DNA damage response.
Conflict of interest statement
Figures

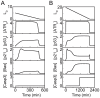
 (A) or 20 (B).
(A) or 20 (B).
 (without DNA repair). (B) Time courses of [ATRp] at
(without DNA repair). (B) Time courses of [ATRp] at  (black), 10 (red), or 20 (blue).
(black), 10 (red), or 20 (blue).
 (black), 10 (red), or 20 (blue). (B) Maximum of [p53p] throughout the cellular response vs.
(black), 10 (red), or 20 (blue). (B) Maximum of [p53p] throughout the cellular response vs.  . (C) Bifurcation diagram of [p53p] vs. the p53-inducible production rate of PTEN,
. (C) Bifurcation diagram of [p53p] vs. the p53-inducible production rate of PTEN,  , at fixed
, at fixed  . (D) Time courses of [p53p] with
. (D) Time courses of [p53p] with  (black) or 0.05 (red).
(black) or 0.05 (red).
 . (B) Time courses of [p21tot] (black), [E2F1] (green), [Apaf-1] (red), and [Casp9] (blue) at
. (B) Time courses of [p21tot] (black), [E2F1] (green), [Apaf-1] (red), and [Casp9] (blue) at  . (C) Bifurcation diagram of [E2F1] vs. the p53-inducible production rate of p21,
. (C) Bifurcation diagram of [E2F1] vs. the p53-inducible production rate of p21,  . (D) Time courses of [p21tot] (black), [E2F1] (blue), [CytoC] (red), and [Casp9] (green) with
. (D) Time courses of [p21tot] (black), [E2F1] (blue), [CytoC] (red), and [Casp9] (green) with  and
and  .
.
 5 (black) or 20 (red). (B) Bifurcation diagram of [p21tot] vs. the p53-inducible production rate of DDB2,
5 (black) or 20 (red). (B) Bifurcation diagram of [p21tot] vs. the p53-inducible production rate of DDB2,  . (C) Time courses of [p21tot] (black), [E2F1] (green), [Bax] (red), and [Casp9] (blue) with
. (C) Time courses of [p21tot] (black), [E2F1] (green), [Bax] (red), and [Casp9] (blue) with  at
at  . (D) Time courses of [Casp9] with the normal parameter setting (i.e.,
. (D) Time courses of [Casp9] with the normal parameter setting (i.e.,  and
and  ) (black),
) (black),  and
and  (blue), or
(blue), or  and
and  (red).
(red).References
-
- Meek DW (2009) Tumour suppression by p53: a role for the DNA damage response? Nat Rev Cancer 9: 714–723. - PubMed
-
- Murray-Zmijewski F, Slee EA, Lu X (2008) A complex barcode underlies the heterogeneous response of p53 to stress. Nat Rev Mol Cell Biol 9: 702–712. - PubMed
-
- Speidel D (2009) Transcription-independent p53 apoptosis: an alternative route to death. Trends Cell Biol 20: 14–24. - PubMed
-
- Lahav G, Rosenfeld N, Sigal A, Geva-Zatorsky N, Levine AJ, et al. (2004) Dynamics of the p53-Mdm2 feedback loop in individual cells. Nat Genet 36: 147–150. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

