Retinal cone photoreceptors of the deer mouse Peromyscus maniculatus: development, topography, opsin expression and spectral tuning
- PMID: 24260509
- PMCID: PMC3829927
- DOI: 10.1371/journal.pone.0080910
Retinal cone photoreceptors of the deer mouse Peromyscus maniculatus: development, topography, opsin expression and spectral tuning
Abstract
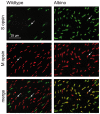
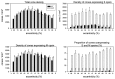
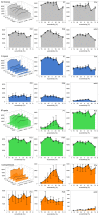
A quantitative analysis of photoreceptor properties was performed in the retina of the nocturnal deer mouse, Peromyscus maniculatus, using pigmented (wildtype) and albino animals. The aim was to establish whether the deer mouse is a more suitable model species than the house mouse for photoreceptor studies, and whether oculocutaneous albinism affects its photoreceptor properties. In retinal flatmounts, cone photoreceptors were identified by opsin immunostaining, and their numbers, spectral types, and distributions across the retina were determined. Rod photoreceptors were counted using differential interference contrast microscopy. Pigmented P. maniculatus have a rod-dominated retina with rod densities of about 450.000/mm(2) and cone densities of 3000-6500/mm(2). Two cone opsins, shortwave sensitive (S) and middle-to-longwave sensitive (M), are present and expressed in distinct cone types. Partial sequencing of the S opsin gene strongly supports UV sensitivity of the S cone visual pigment. The S cones constitute a 5-15% minority of the cones. Different from house mouse, S and M cone distributions do not have dorsoventral gradients, and coexpression of both opsins in single cones is exceptional (<2% of the cones). In albino P. maniculatus, rod densities are reduced by approximately 40% (270.000/mm(2)). Overall, cone density and the density of cones exclusively expressing S opsin are not significantly different from pigmented P. maniculatus. However, in albino retinas S opsin is coexpressed with M opsin in 60-90% of the cones and therefore the population of cones expressing only M opsin is significantly reduced to 5-25%. In conclusion, deer mouse cone properties largely conform to the general mammalian pattern, hence the deer mouse may be better suited than the house mouse for the study of certain basic cone properties, including the effects of albinism on cone opsin expression.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

