Development of NMDAR antagonists with reduced neurotoxic side effects: a study on GK11
- PMID: 24260528
- PMCID: PMC3834252
- DOI: 10.1371/journal.pone.0081004
Development of NMDAR antagonists with reduced neurotoxic side effects: a study on GK11
Abstract
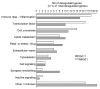
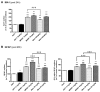
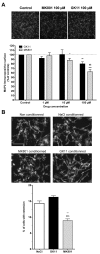
The NMDAR glutamate receptor subtype mediates various vital physiological neuronal functions. However, its excessive activation contributes to neuronal damage in a large variety of acute and chronic neurological disorders. NMDAR antagonists thus represent promising therapeutic tools that can counteract NMDARs' overactivation. Channel blockers are of special interest since they are use-dependent, thus being more potent at continuously activated NMDARs, as may be the case in pathological conditions. Nevertheless, it has been established that NMDAR antagonists, such as MK801, also have unacceptable neurotoxic effects. Presently only Memantine is considered a safe NMDAR antagonist and is used clinically. It has recently been speculated that antagonists that preferentially target extrasynaptic NMDARs would be less toxic. We previously demonstrated that the phencyclidine derivative GK11 preferentially inhibits extrasynaptic NMDARs. We thus anticipated that this compound would be safer than other known NMDAR antagonists. In this study we used whole-genome profiling of the rat cingulate cortex, a brain area that is particularly sensitive to NMDAR antagonists, to compare the potential adverse effects of GK11 and MK801. Our results showed that in contrast to GK11, the transcriptional profile of MK801 is characterized by a significant upregulation of inflammatory and stress-response genes, consistent with its high neurotoxicity. In addition, behavioural and immunohistochemical analyses confirmed marked inflammatory reactions (including astrogliosis and microglial activation) in MK801-treated, but not GK11-treated rats. Interestingly, we also showed that GK11 elicited less inflammation and neuronal damage, even when compared to Memantine, which like GK11, preferentially inhibits extrasynaptic NMDAR. As a whole, our study suggests that GK11 may be a more attractive therapeutic alternative in the treatment of CNS disorders characterized by the overactivation of glutamate receptors.
Conflict of interest statement
Figures


Similar articles
-
Comparison of the pharmacological properties of GK11 and MK801, two NMDA receptor antagonists: towards an explanation for the lack of intrinsic neurotoxicity of GK11.J Neurochem. 2007 Nov;103(4):1682-96. doi: 10.1111/j.1471-4159.2007.04925.x. Epub 2007 Oct 1. J Neurochem. 2007. PMID: 17908242
-
Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses.J Neurosci. 2010 Aug 18;30(33):11246-50. doi: 10.1523/JNEUROSCI.2488-10.2010. J Neurosci. 2010. PMID: 20720132 Free PMC article.
-
NMDA Receptor Antagonist MK801 Protects Against 1-Bromopropane-Induced Cognitive Dysfunction.Neurosci Bull. 2019 Apr;35(2):347-361. doi: 10.1007/s12264-018-0321-8. Epub 2018 Dec 19. Neurosci Bull. 2019. PMID: 30569431 Free PMC article.
-
The chemical biology of clinically tolerated NMDA receptor antagonists.J Neurochem. 2006 Jun;97(6):1611-26. doi: 10.1111/j.1471-4159.2006.03991.x. J Neurochem. 2006. PMID: 16805772 Review.
-
Targeting NMDA receptors with an antagonist is a promising therapeutic strategy for treating neurological disorders.Behav Brain Res. 2024 Aug 24;472:115173. doi: 10.1016/j.bbr.2024.115173. Epub 2024 Aug 3. Behav Brain Res. 2024. PMID: 39097148 Review.
Cited by
-
Serum Reactive Antibodies against the N-Methyl-D-Aspartate Receptor NR2 Subunit-Could They Act as Potential Biomarkers?Int J Mol Sci. 2023 Nov 10;24(22):16170. doi: 10.3390/ijms242216170. Int J Mol Sci. 2023. PMID: 38003360 Free PMC article.
-
The synergistic effect of treatment with triptolide and MK-801 in the rat neuropathic pain model.Mol Pain. 2017 Jan-Dec;13:1744806917746564. doi: 10.1177/1744806917746564. Epub 2017 Nov 22. Mol Pain. 2017. PMID: 29166839 Free PMC article.
-
Gacyclidine improves the survival and reduces motor deficits in a mouse model of amyotrophic lateral sclerosis.Front Cell Neurosci. 2013 Dec 27;7:280. doi: 10.3389/fncel.2013.00280. eCollection 2013. Front Cell Neurosci. 2013. PMID: 24409117 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

