The LIM-only protein FHL2 attenuates lung inflammation during bleomycin-induced fibrosis
- PMID: 24260575
- PMCID: PMC3832604
- DOI: 10.1371/journal.pone.0081356
The LIM-only protein FHL2 attenuates lung inflammation during bleomycin-induced fibrosis
Abstract
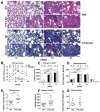
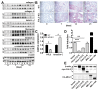
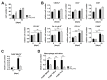
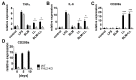
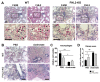
Fibrogenesis is usually initiated when regenerative processes have failed and/or chronic inflammation occurs. It is characterised by the activation of tissue fibroblasts and dysregulated synthesis of extracellular matrix proteins. FHL2 (four-and-a-half LIM domain protein 2) is a scaffolding protein that interacts with numerous cellular proteins, regulating signalling cascades and gene transcription. It is involved in tissue remodelling and tumour progression. Recent data suggest that FHL2 might support fibrogenesis by maintaining the transcriptional expression of alpha smooth muscle actin and the excessive synthesis and assembly of matrix proteins in activated fibroblasts. Here, we present evidence that FHL2 does not promote bleomycin-induced lung fibrosis, but rather suppresses this process by attenuating lung inflammation. Loss of FHL2 results in increased expression of the pro-inflammatory matrix protein tenascin C and downregulation of the macrophage activating C-type lectin receptor DC-SIGN. Consequently, FHL2 knockout mice developed a severe and long-lasting lung pathology following bleomycin administration due to enhanced expression of tenascin C and impaired activation of inflammation-resolving macrophages.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

