Antagonist properties of Conus parius peptides on N-methyl-D-aspartate receptors and their effects on CREB signaling
- PMID: 24260577
- PMCID: PMC3832412
- DOI: 10.1371/journal.pone.0081405
Antagonist properties of Conus parius peptides on N-methyl-D-aspartate receptors and their effects on CREB signaling
Abstract
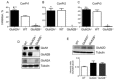
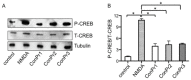
Three members of a family of small neurotoxic peptides from the venom of Conus parius, conantokins (Con) Pr1, Pr2, and Pr3, function as antagonists of N-methyl-D-aspartate receptors (NMDAR). We report structural characterizations of these synthetic peptides, and also demonstrate their antagonistic properties toward ion flow through NMDAR ion channels in primary neurons. ConPr1 and ConPr2 displayed moderate increases in α-helicity after addition of Mg(2+). Native apo-ConPr3 possessed an α-helical conformation, and the helicity increased only slightly on addition of Mg(2+). Additionally, these peptides diminished NMDA/Gly-mediated currents and intracellular Ca(2+) (iCa(2+)) influx in mature rat primary hippocampal neurons. Electrophysiological data showed that these peptides displayed slower antagonistic properties toward the NMDAR than conantokins from other species of cone snails, e.g., ConT and ConG. Furthermore, to demonstrate selectivity of the C. parius-derived conantokins towards specific NMDAR subunits, cortical neurons from GluN2A(-/-) and GluN2B(-/-) mice were utilized. Robust inhibition of NMDAR-mediated stimulation in GluN2A(-/-)-derived mouse neurons, as compared to those isolated from GluN2B(-/-)-mouse brains, was observed, suggesting a greater selectivity of these antagonists towards the GluN2B subunit. These C. parius conantokins mildly inhibited NMDAR-induced phosphorylation of CREB at Ser(133), suggesting that the peptides modulated iCa(2+) entry and, thereby, activation of CREB, a transcription factor that is required for maintaining long-term synaptic activity. Our data mechanistically show that while these peptides effectively antagonize NMDAR-directed current and iCa(2+) influx, receptor-coupled CREB signaling is maintained. The consequence of sustained CREB signaling is improved neuronal plasticity and survival during neuropathologies.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

