N-nitrosomelatonin enhances photic synchronization of mammalian circadian rhythms
- PMID: 24261470
- PMCID: PMC4024459
- DOI: 10.1111/jnc.12613
N-nitrosomelatonin enhances photic synchronization of mammalian circadian rhythms
Abstract
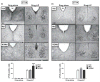
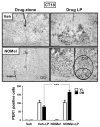
Most physiological processes in mammals are synchronized to the daily light:dark cycle by a circadian clock located in the hypothalamic suprachiasmatic nucleus. Signal transduction of light-induced phase advances of the clock is mediated through a neuronal nitric oxide synthase-guanilyl cyclase pathway. We have employed a novel nitric oxide-donor, N-nitrosomelatonin, to enhance the photic synchronization of circadian rhythms in hamsters. The intraperitoneal administration of this drug before a sub-saturating light pulse at circadian time 18 generated a twofold increase of locomotor rhythm phase-advances, having no effect over saturating light pulses. This potentiation was also obtained even when inhibiting suprachiasmatic nitric oxide synthase activity. However, N-nitrosomelatonin had no effect on light-induced phase delays at circadian time 14. The photic-enhancing effects were correlated with an increased suprachiasmatic immunoreactivity of FBJ murine osteosarcoma viral oncogene and period1. Moreover, in vivo nitric oxide release by N-nitrosomelatonin was verified by measuring nitrate and nitrite levels in suprachiasmatic nuclei homogenates. The compound also accelerated resynchronization to an abrupt 6-h advance in the light:dark cycle (but not resynchronization to a 6-h delay). Here, we demonstrate the chronobiotic properties of N-nitrosomelatonin, emphasizing the importance of nitric oxide-mediated transduction for circadian phase advances.
Keywords: N-nitrosomelatonin; circadian; jet lag; nitric oxide; suprachiasmatic nucleus.
© 2013 International Society for Neurochemistry.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures





References
-
- Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain research. 2001;916:172–191. - PubMed
-
- Agostino PV, Ferreyra GA, Murad AD, Watanabe Y, Golombek DA. Diurnal, circadian and photic regulation of calcium/calmodulin-dependent kinase II and neuronal nitric oxide synthase in the hamster suprachiasmatic nuclei. Neurochemistry international. 2004;44:617–625. - PubMed
-
- Aida R, Moriya T, Araki M, Akiyama M, Wada K, Wada E, Shibata S. Gastrin-releasing peptide mediates photic entrainable signals to dorsal subsets of suprachiasmatic nucleus via induction of Period gene in mice. Molecular pharmacology. 2002;61:26–34. - PubMed
-
- Akiyama M, Kouzu Y, Takahashi S, et al. Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1999;19:1115–1121. - PMC - PubMed
-
- Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC. MPer1 and mper2 are essential for normal resetting of the circadian clock. Journal of biological rhythms. 2001;16:100–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

