Influenza A penetrates host mucus by cleaving sialic acids with neuraminidase
- PMID: 24261589
- PMCID: PMC3842836
- DOI: 10.1186/1743-422X-10-321
Influenza A penetrates host mucus by cleaving sialic acids with neuraminidase
Abstract
Background: Influenza A virus (IAV) neuraminidase (NA) cleaves sialic acids (Sias) from glycans. Inhibiting NA with oseltamivir suppresses both viral infection, and viral release from cultured human airway epithelial cells. The role of NA in viral exit is well established: it releases budding virions by cleaving Sias from glycoconjugates on infected cells and progeny virions. The role of NA in viral entry remains unclear. Host respiratory epithelia secrete a mucus layer rich in heavily sialylated glycoproteins; these could inhibit viral entry by mimicking sialylated receptors on the cell surface. It has been suggested that NA allows influenza to penetrate the mucus by cleaving these sialylated decoys, but the exact mechanism is not yet established.
Methods: We tested IAV interaction with secreted mucus using frozen human trachea/bronchus tissue sections, and bead-bound purified human salivary mucins (HSM) and purified porcine submaxillary mucins (PSM). The protective effect of mucus was analyzed using MDCK cells coated with purified HSM and PSM with known Sia content. Oseltamivir was used to inhibit NA activity, and the fluorescent reporter substrate, 4MU-Neu5Ac, was used to quantify NA activity.
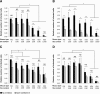
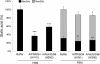
Results: IAV binds to the secreted mucus layer of frozen human trachea/bronchus tissues in a Sia dependent manner. HSM inhibition of IAV infection is Sia dose-dependent, but PSM cannot inhibit infection of underlying cells. HSM competitively inhibits NA cleavage of 4MU-Neu5Ac, reporter substrate. Human IAV effectively cleaves Sias from HSM but not from PSM, and binds to HSM but not to PSM.
Conclusion: IAV interacts with human mucus on frozen tissue sections and mucus-coated beads. Inhibition of IAV infection by sialylated human mucus is dose-dependent, and enhanced when NA is inhibited with oseltamivir. Thus NA cleaves sialylated decoys during initial stages of infection. Understanding IAV interactions with host mucins is a promising new avenue for drug development.
Figures



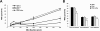
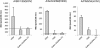
References
-
- Andrewes CH. Nomenclature of viruses. Nature. 1954;10:620–621. - PubMed
-
- Burnet FM. Mucoproteins in relation to virus action. Physiol Rev. 1951;10:131–150. - PubMed
-
- Duez JM, Sixt N, Pechinot A. Influenza virus infection: don’t forget the role of the mucociliary system! J Antimicrob Chemother. 2009;10:421–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

