Anti-dementia drugs and changes in gait: a pre-post quasi-experimental pilot study
- PMID: 24261605
- PMCID: PMC3898226
- DOI: 10.1186/1471-2377-13-184
Anti-dementia drugs and changes in gait: a pre-post quasi-experimental pilot study
Abstract
Background: Anti-dementia drugs may improve gait performance. No comparison between acetylcholinesterase inhibitors (CEIs) and memantine-related changes in gait variability has been reported. The objectives of this study were to 1) quantify and compare the mean values and coefficients of variation (CoV) of stride time in demented patients with Alzheimer's disease and related disorders (ADRD) before and after the use of CEIs or memantine, and in age- and gender-matched controls patients with ADRD using no anti-dementia drugs; and 2) to determine whether changes in CoV of stride time differed between CEIs or memantine.
Methods: A total of 120 demented patients with mild-to-moderate ADRD were prospectively included in this pre-post quasi-experimental study with two intervention groups (43 patients taking CEIs, and 41 taking memantine) and a control group (36 age- and gender matched patients without any anti-dementia drugs). CoV of stride time and walking speed were measured with GAITRite® system while usual walking at steady state. Age, gender, number of drugs daily taken, use of psychoactive drugs, body mass index and time between the two visits were also recorded.
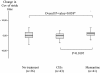
Results: There was no difference between groups for the time between baseline and follow-up assessments (232.9 ± 103.7 days for patients without anti-dementia drugs, 220.0 ± 67.5 days for patients with CEIs, 186.7 ± 96.2 days for patients with memantine, P = 0.062). Patients with memantine had a lower (i.e., better) CoV of stride time at follow-up assessment compared to those with CEIs (4.2 ± 2.4% versus 5.8 ± 4.2%, P = 0.010). Patients with memantine had a greater decrease in CoV of stride time compared to those with CEIs (-1.90% versus 0.93%, P = 0.010) and mixed-effects linear regressions showed that this decrease was specifically explained by memantine (P = 0.028).
Conclusions: Our results showed that patients with ADRD and treated with memantine, but not those with CEIs, decreased their gait variability, and thus improved their gait safety (Trial registration number: NCT01315704).
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

