Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies
- PMID: 24262108
- PMCID: PMC4176484
- DOI: 10.1186/2045-8118-10-33
Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies
Abstract
Background: Reliable human in vitro blood-brain barrier (BBB) models suitable for high-throughput screening are urgently needed in early drug discovery and development for assessing the ability of promising bioactive compounds to overcome the BBB. To establish an improved human in vitro BBB model, we compared four currently available and well characterized immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, with respect to barrier tightness and paracellular permeability. Co-culture systems using immortalized human astrocytes (SVG-A cell line) and immortalized human pericytes (HBPCT cell line) were designed with the aim of positively influencing barrier tightness.
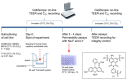
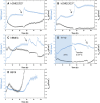
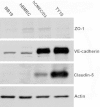
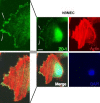
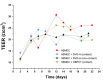
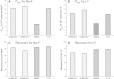
Methods: Tight junction (TJ) formation was assessed by transendothelial electrical resistance (TEER) measurements using a conventional epithelial voltohmmeter (EVOM) and an automated CellZscope system which records TEER and cell layer capacitance (CCL) in real-time.Paracellular permeability was assessed using two fluorescent marker compounds with low BBB penetration (sodium fluorescein (Na-F) and lucifer yellow (LY)). Conditions were optimized for each endothelial cell line by screening a series of 24-well tissue culture inserts from different providers. For hBMEC cells, further optimization was carried out by varying coating material, coating procedure, cell seeding density, and growth media composition. Biochemical characterization of cell type-specific transmembrane adherens junction protein VE-cadherin and of TJ proteins ZO-1 and claudin-5 were carried out for each endothelial cell line. In addition, immunostaining for ZO-1 in hBMEC cell line was performed.
Results: The four cell lines all expressed the endothelial cell type-specific adherens junction protein VE-cadherin. The TJ protein ZO-1 was expressed in hCMEC/D3 and in hBMEC cells. ZO-1 expression could be confirmed in hBMEC cells by immunocytochemical staining. Claudin-5 expression was detected in hCMEC/D3, TY10, and at a very low level in hBMEC cells. Highest TEER values and lowest paracellular permeability for Na-F and LY were obtained with mono-cultures of hBMEC cell line when cultivated on 24-well tissue culture inserts from Greiner Bio-one® (transparent PET membrane, 3.0 μm pore size). In co-culture models with SVG-A and HBPCT cells, no increase of TEER could be observed, suggesting that none of the investigated endothelial cell lines responded positively to stimuli from immortalized astrocytic or pericytic cells.
Conclusions: Under the conditions examined in our experiments, hBMEC proved to be the most suitable human cell line for an in vitro BBB model concerning barrier tightness in a 24-well mono-culture system intended for higher throughput. This BBB model is being validated with several compounds (known to cross or not to cross the BBB), and will potentially be selected for the assessment of BBB permeation of bioactive natural products.
Figures


References
-
- Di L, Rong H, Feng B. Demystifying brain penetration in central nervous system drug discovery. J Med Chem. 2013;56:A–K. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

