A multisite randomized controlled trial of brief intervention to reduce drinking in the trauma care setting: how brief is brief?
- PMID: 24263324
- PMCID: PMC3984362
- DOI: 10.1097/SLA.0000000000000339
A multisite randomized controlled trial of brief intervention to reduce drinking in the trauma care setting: how brief is brief?
Abstract
Objective: Determine the efficacy of 3 brief intervention strategies that address heavy drinking among injured patients.
Background: The content or structure of brief interventions most effective at reducing alcohol misuse after traumatic injury is not known.
Methods: Injured patients from 3 trauma centers were screened for heavy drinking and randomly assigned to brief advice (n = 200), brief motivational intervention (BMI) (n = 203), or BMI plus a telephone booster using personalized feedback or BMI + B (n = 193). Among those randomly assigned, 57% met criteria for moderate to severe alcohol problems. The primary drinking outcomes were assessed at 3, 6, and 12 months.
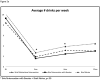
Results: Compared with brief advice and BMI, BMI + B showed significant reductions in the number of standard drinks consumed per week at 3 (Δ adjusted means: -1.22, 95% confidence interval [CI]: -0.99, approximately -1.49, P = 0.01) and 6 months (Δ adjusted means: -1.42, 95% CI: -1.14, approximately -1.76, P = 0.02), percent days of heavy drinking at 6 months (Δ adjusted means: -5.90, 95% CI: -11.40, approximately -0.40, P = 0.04), maximum number of standard drinks consumed in 1 day at 3 (Δ adjusted means: -1.38, 95% CI: -1.18, approximately -1.62, P = 0.003) and 12 months (Δ adjusted means: -1.71, 95% CI: -1.47, approximately -1.99, P = 0.02), and number of standard drinks consumed per drinking day at 3 (Δ adjusted means: -1.49, 95% CI: -1.35, approximately -1.65, P = 0.002) and 6 months (Δ adjusted means: -1.28, 95% CI: -1.17, approximately -1.40, P = 0.01).
Conclusions: Brief interventions based on motivational interviewing with a telephone booster using personalized feedback were most effective at achieving reductions in alcohol intake across the 3 trauma centers.
Trial registration: ClinicalTrials.gov NCT00428181.
Conflict of interest statement
Figures



References
-
- Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States, 2000. JAMA. 2004;29:1238–45. - PubMed
-
- McKenna MT, Michaud CM, Murray CJ, et al. Assessing the burden of disease in the United States using disability-adjusted life years. Am J Prev Med. 2005;28:415–23. - PubMed
-
- Room R, Babor T, Rehm J. Alcohol and public health. Lancet. 2005;365:519–30. - PubMed
-
- Solberg LI, Maciosek MV, Edwards NM. Primary care intervention to reduce alcohol misuse ranking its health impact and cost effectiveness. Am J Prev Med. 2008;34:143–52. - PubMed
-
- American College of Surgeons Committee on Trauma. Resources for optimal care of the injured patient: 2006. Chicago: American College of Surgeons; 1993.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

