Cell intrinsic and extrinsic factors synergize in mice with haploinsufficiency for Tp53, and two human del(5q) genes, Egr1 and Apc
- PMID: 24264229
- PMCID: PMC3888289
- DOI: 10.1182/blood-2013-05-506568
Cell intrinsic and extrinsic factors synergize in mice with haploinsufficiency for Tp53, and two human del(5q) genes, Egr1 and Apc
Abstract
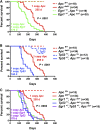
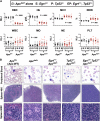
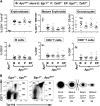
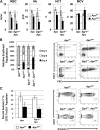
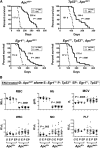
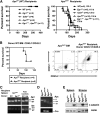
Therapy-related myeloid neoplasms (t-MN) are a late complication of the successful use of cytotoxic therapy for patients with cancer. A heterozygous deletions of the long arm of chromosome 5 [del(5q)], observed in 40% of patients, is associated with prior exposure to alkylating agents, and a high frequency of TP53 loss or mutation. In previous studies, we demonstrated that haploinsufficiency of 2 del(5q) genes, Egr1, and Apc, individually play a role in the pathogenesis of hematologic disease in mice. We now show that loss of one copy of Egr1 or Tp53 in an Apc haploinsufficient background (Apc (del/+)) accelerated the development of a macrocytic anemia with monocytosis, early features of t-MN. The development of anemia was significantly accelerated by treatment of mice with the alkylating agent, N-ethyl-N-nitrosourea (ENU), regardless of the levels of expression of Egr1 and Tp53. Transplantation of either wild type; Egr1(+/-); Tp53(+/-); Apc(del/+); or Egr1(+/-), Apc(del/+) bone marrow cells into lethally irradiated Apc(del/+) recipients resulted in rapid development of anemia that was further accelerated by administration of ENU to recipients, demonstrating that the Apc(del/+)-induced anemia was cell extrinsic and potentiated by ENU mutagenesis. These data emphasize the synergistic role of cell intrinsic and cell extrinsic (microenvironment) factors in the pathogenesis of t-MN, and raise awareness of the deleterious effects of cytotoxic therapy on the stromal microenvironment.
Figures

Similar articles
-
Haploinsufficiency of del(5q) genes, Egr1 and Apc, cooperate with Tp53 loss to induce acute myeloid leukemia in mice.Blood. 2014 Feb 13;123(7):1069-78. doi: 10.1182/blood-2013-07-517953. Epub 2013 Dec 31. Blood. 2014. PMID: 24381225 Free PMC article.
-
Haploinsufficiency of Apc leads to ineffective hematopoiesis.Blood. 2010 Apr 29;115(17):3481-8. doi: 10.1182/blood-2009-11-251835. Epub 2010 Jan 11. Blood. 2010. PMID: 20065296 Free PMC article.
-
Haploinsufficiency of EGR1, a candidate gene in the del(5q), leads to the development of myeloid disorders.Blood. 2007 Jul 15;110(2):719-26. doi: 10.1182/blood-2007-01-068809. Epub 2007 Apr 9. Blood. 2007. PMID: 17420284 Free PMC article.
-
Molecular dissection of the 5q deletion in myelodysplastic syndrome.Semin Oncol. 2011 Oct;38(5):621-6. doi: 10.1053/j.seminoncol.2011.04.010. Semin Oncol. 2011. PMID: 21943668 Free PMC article. Review.
-
Deletion 5q MDS: molecular and therapeutic implications.Best Pract Res Clin Haematol. 2013 Dec;26(4):365-75. doi: 10.1016/j.beha.2013.10.013. Epub 2013 Oct 16. Best Pract Res Clin Haematol. 2013. PMID: 24507813 Review.
Cited by
-
BRCA1 and TP53 codeficiency causes a PARP inhibitor-sensitive erythroproliferative neoplasm.JCI Insight. 2022 Dec 22;7(24):e158257. doi: 10.1172/jci.insight.158257. JCI Insight. 2022. PMID: 36346676 Free PMC article.
-
Haploinsufficiency of del(5q) genes, Egr1 and Apc, cooperate with Tp53 loss to induce acute myeloid leukemia in mice.Blood. 2014 Feb 13;123(7):1069-78. doi: 10.1182/blood-2013-07-517953. Epub 2013 Dec 31. Blood. 2014. PMID: 24381225 Free PMC article.
-
Aberrant overexpression of CD14 on granulocytes sensitizes the innate immune response in mDia1 heterozygous del(5q) MDS.Blood. 2014 Jul 31;124(5):780-90. doi: 10.1182/blood-2014-01-552463. Epub 2014 Jun 2. Blood. 2014. PMID: 24891322 Free PMC article. Clinical Trial.
-
Therapy-related myeloid neoplasms: when genetics and environment collide.Nat Rev Cancer. 2017 Aug 24;17(9):513-527. doi: 10.1038/nrc.2017.60. Nat Rev Cancer. 2017. PMID: 28835720 Free PMC article. Review.
-
Inhibition of WNT signaling in the bone marrow niche prevents the development of MDS in the Apcdel/+ MDS mouse model.Blood. 2017 Jun 1;129(22):2959-2970. doi: 10.1182/blood-2016-08-736454. Epub 2017 Mar 27. Blood. 2017. PMID: 28348148 Free PMC article.
References
-
- Pedersen-Bjergaard J, Andersen MK, Andersen MT, Christiansen DH. Genetics of therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2008;22(2):240–248. - PubMed
-
- Smith SM, Le Beau MM, Huo D, et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood. 2003;102(1):43–52. - PubMed
-
- Boultwood J, Pellagatti A, McKenzie AN, Wainscoat JS. Advances in the 5q- syndrome. Blood. 2010;116(26):5803–5811. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

