Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy
- PMID: 24265155
- PMCID: PMC3936420
- DOI: 10.1158/2159-8290.CD-13-0642
Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy
Abstract
BRAF inhibitors elicit rapid antitumor responses in the majority of patients with BRAF(V600)-mutant melanoma, but acquired drug resistance is almost universal. We sought to identify the core resistance pathways and the extent of tumor heterogeneity during disease progression. We show that mitogen-activated protein kinase reactivation mechanisms were detected among 70% of disease-progressive tissues, with RAS mutations, mutant BRAF amplification, and alternative splicing being most common. We also detected PI3K-PTEN-AKT-upregulating genetic alterations among 22% of progressive melanomas. Distinct molecular lesions in both core drug escape pathways were commonly detected concurrently in the same tumor or among multiple tumors from the same patient. Beyond harboring extensively heterogeneous resistance mechanisms, melanoma regrowth emerging from BRAF inhibitor selection displayed branched evolution marked by altered mutational spectra/signatures and increased fitness. Thus, melanoma genomic heterogeneity contributes significantly to BRAF inhibitor treatment failure, implying upfront, cotargeting of two core pathways as an essential strategy for durable responses.
Figures
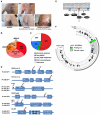
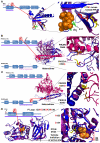
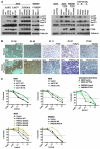
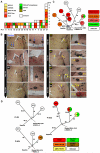
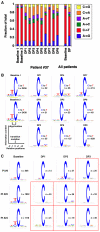
Comment in
-
Therapeutics: delving deeper into resistance.Nat Rev Cancer. 2014 Jan;14(1):7. doi: 10.1038/nrc3653. Nat Rev Cancer. 2014. PMID: 24505615 No abstract available.
References
-
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002 Jun 27;417(6892):949–54. - PubMed
-
- Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012 Jul 28;380(9839):358–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

