Timing is everything: GTPase regulation in phototransduction
- PMID: 24265205
- PMCID: PMC3837634
- DOI: 10.1167/iovs.13-13281
Timing is everything: GTPase regulation in phototransduction
Abstract
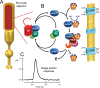
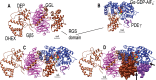
As the molecular mechanisms of vertebrate phototransduction became increasingly clear in the 1980s, a persistent problem was the discrepancy between the slow GTP hydrolysis catalyzed by the phototransduction G protein, transducin, and the much more rapid physiological recovery of photoreceptor cells from light stimuli. Beginning with a report published in 1989, a series of studies revealed that transducin GTPase activity could approach the rate needed to explain physiological recovery kinetics in the presence of one or more factors present in rod outer segment membranes. One by one, these factors were identified, beginning with PDEγ, the inhibitory subunit of the cGMP phosphodiesterase activated by transducin. There followed the discovery of the crucial role played by the regulator of G protein signaling, RGS9, a member of a ubiquitous family of GTPase-accelerating proteins, or GAPs, for heterotrimeric G proteins. Soon after, the G protein β isoform Gβ5 was identified as an obligate partner subunit, followed by the discovery or R9AP, a transmembrane protein that anchors the RGS9 GAP complex to the disk membrane, and is essential for the localization, stability, and activity of this complex in vivo. The physiological importance of all of the members of this complex was made clear first by knockout mouse models, and then by the discovery of a human visual defect, bradyopsia, caused by an inherited deficiency in one of the GAP components. Further insights have been gained by high-resolution crystal structures of subcomplexes, and by extensive mechanistic studies both in vitro and in animal models.
Figures

References
-
- Burns ME, Baylor DA. Activation, deactivation, and adaptation in vertebrate photoreceptor cells. Annu Rev Neurosci. 2001; 24: 779–805 - PubMed
-
- Fain GL, Matthews HR, Cornwall MC, Koutalos Y. Adaptation in vertebrate photoreceptors. Physiol Rev. 2001; 81: 117–151 - PubMed
-
- Arshavsky VY, Lamb TD, Pugh EN Jr. G proteins and phototransduction. Annu Rev Physiol. 2002; 64: 153–187 - PubMed
-
- Arshavsky VY, Dizhoor AM, Shestakova IK, Philippov P. The effect of rhodopsin phosphorylation on the light-dependent activation of phosphodiesterase from bovine rod outer segments. FEBS Lett. 1985; 181: 264–266 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

