Mechanical properties of respiratory muscles
- PMID: 24265238
- PMCID: PMC3977503
- DOI: 10.1002/cphy.c130003
Mechanical properties of respiratory muscles
Abstract
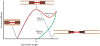
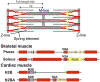
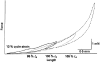
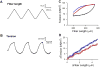
Striated respiratory muscles are necessary for lung ventilation and to maintain the patency of the upper airway. The basic structural and functional properties of respiratory muscles are similar to those of other striated muscles (both skeletal and cardiac). The sarcomere is the fundamental organizational unit of striated muscles and sarcomeric proteins underlie the passive and active mechanical properties of muscle fibers. In this respect, the functional categorization of different fiber types provides a conceptual framework to understand the physiological properties of respiratory muscles. Within the sarcomere, the interaction between the thick and thin filaments at the level of cross-bridges provides the elementary unit of force generation and contraction. Key to an understanding of the unique functional differences across muscle fiber types are differences in cross-bridge recruitment and cycling that relate to the expression of different myosin heavy chain isoforms in the thick filament. The active mechanical properties of muscle fibers are characterized by the relationship between myoplasmic Ca2+ and cross-bridge recruitment, force generation and sarcomere length (also cross-bridge recruitment), external load and shortening velocity (cross-bridge cycling rate), and cross-bridge cycling rate and ATP consumption. Passive mechanical properties are also important reflecting viscoelastic elements within sarcomeres as well as the extracellular matrix. Conditions that affect respiratory muscle performance may have a range of underlying pathophysiological causes, but their manifestations will depend on their impact on these basic elemental structures.
© 2013 American Physiological Society. Compr Physiol 3:1533-1567, 2013.
Figures




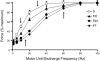
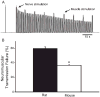
References
-
- Adelman WJ, Palti Y, Senft JP. Potassium ion accumulation in a periaxonal space and its effect on the measurement of membrane potassium ion conductance. J Membr Biol. 1973;13:387–410. - PubMed
-
- Aldrich TK, Shander A, Chaudhry I, Nagashima H. Fatigue of isolated rat diaphragm: role of impaired neuromuscular transmission. J Appl Physiol. 1986;61:1077–1083. - PubMed
-
- Aldrich TK. Transmission fatigue of the rabbit diaphragm. Respir Physiol. 1987;69:307–319. - PubMed
-
- Allan DW, Greer JJ. Embryogenesis of the phrenic nerve and diaphragm in the fetal rat. J Comp Neurol. 1997;382:459–468. - PubMed
-
- Allen DG. Skeletal muscle function: role of ionic changes in fatigue, damage and disease. Clin Exp Pharmacol Physiol. 2004;31:485–493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

