Melatonin action in a midbrain vocal-acoustic network
- PMID: 24265429
- PMCID: PMC3966919
- DOI: 10.1242/jeb.096669
Melatonin action in a midbrain vocal-acoustic network
Abstract
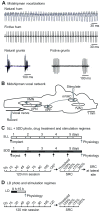
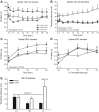
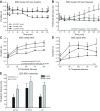
Melatonin is a well-documented time-keeping hormone that can entrain an individual's physiology and behavior to the day-night cycle, though surprisingly little is known about its influence on the neural basis of social behavior, including vocalization. Male midshipman fish (Porichthys notatus) produce several call types distinguishable by duration and by daily and seasonal cycles in their production. We investigated melatonin's influence on the known nocturnal- and breeding season-dependent increase in excitability of the midshipman's vocal network (VN) that directly patterns natural calls. VN output is readily recorded from the vocal nerve as a 'fictive call'. Five days of constant light significantly increased stimulus threshold levels for calls electrically evoked from vocally active sites in the medial midbrain, supporting previous findings that light suppresses VN excitability, while 2-iodomelatonin (2-IMel; a melatonin analog) implantation decreased threshold. 2-IMel also increased fictive call duration evoked from medial sites as well as lateral midbrain sites that produced several-fold longer calls irrespective of photoregime or drug treatment. When stimulus intensity was incrementally increased, 2-IMel increased duration only at lateral sites, suggesting that melatonin action is stronger in the lateral midbrain. For animals receiving 5 days of constant darkness, known to increase VN excitability, systemic injections of either of two mammalian melatonin receptor antagonists increased threshold and decreased duration for calls evoked from medial sites. Our results demonstrate melatonin modulation of VN excitability and suggest that social context-dependent call types differing in duration may be determined by neuro-hormonal action within specific regions of a midbrain vocal-acoustic network.
Keywords: Melatonin; Midshipman fish; Periaqueductal gray; Vocalization.
Figures


Similar articles
-
Melatonin receptor expression in vocal, auditory, and neuroendocrine centers of a highly vocal fish, the plainfin midshipman (Porichthys notatus).J Comp Neurol. 2019 Jun 1;527(8):1362-1377. doi: 10.1002/cne.24629. Epub 2019 Jan 22. J Comp Neurol. 2019. PMID: 30620047
-
"Singing" Fish Rely on Circadian Rhythm and Melatonin for the Timing of Nocturnal Courtship Vocalization.Curr Biol. 2016 Oct 10;26(19):2681-2689. doi: 10.1016/j.cub.2016.07.079. Epub 2016 Sep 22. Curr Biol. 2016. PMID: 27666972
-
Reproductive and diurnal rhythms regulate vocal motor plasticity in a teleost fish.J Exp Biol. 2009 Oct;212(Pt 20):3252-62. doi: 10.1242/jeb.032748. J Exp Biol. 2009. PMID: 19801430 Free PMC article.
-
Adaptive hearing in the vocal plainfin midshipman fish: getting in tune for the breeding season and implications for acoustic communication.Integr Zool. 2009 Mar;4(1):33-42. doi: 10.1111/j.1749-4877.2008.00133.x. Integr Zool. 2009. PMID: 21392275 Review.
-
A tale of two males: Behavioral and neural mechanisms of alternative reproductive tactics in midshipman fish.Horm Behav. 2024 May;161:105507. doi: 10.1016/j.yhbeh.2024.105507. Epub 2024 Mar 12. Horm Behav. 2024. PMID: 38479349 Review.
Cited by
-
Midbrain node for context-specific vocalisation in fish.Nat Commun. 2024 Jan 2;15(1):189. doi: 10.1038/s41467-023-43794-y. Nat Commun. 2024. PMID: 38167237 Free PMC article.
-
Timing reproduction in teleost fish: cues and mechanisms.Curr Opin Neurobiol. 2016 Jun;38:57-62. doi: 10.1016/j.conb.2016.02.006. Epub 2016 Mar 5. Curr Opin Neurobiol. 2016. PMID: 26952366 Free PMC article. Review.
-
Neuroendocrine control of seasonal plasticity in the auditory and vocal systems of fish.Front Neuroendocrinol. 2015 Apr;37:129-45. doi: 10.1016/j.yfrne.2014.08.002. Epub 2014 Aug 26. Front Neuroendocrinol. 2015. PMID: 25168757 Free PMC article. Review.
-
Neural transcriptome reveals molecular mechanisms for temporal control of vocalization across multiple timescales.BMC Genomics. 2015 May 27;16(1):408. doi: 10.1186/s12864-015-1577-2. BMC Genomics. 2015. PMID: 26014649 Free PMC article.
-
Galanin immunoreactivity is sexually polymorphic in neuroendocrine and vocal-acoustic systems in a teleost fish.J Comp Neurol. 2020 Feb 15;528(3):433-452. doi: 10.1002/cne.24765. Epub 2019 Sep 18. J Comp Neurol. 2020. PMID: 31469908 Free PMC article.
References
-
- Aarseth J. J., Froiland E., Jorgensen E. H. (2010). Melatonin implantation during spring and summer does not affect the seasonal rhythm of feeding in anadromous arctic charr (Salvelinus alpinus). Polar Biol. 33, 379-388
-
- Alvariño J. M. R., Rebollar P. G., Olemdo M., Alvarez-Blázquez B., Peleteiro J. B. (2001). Effects of melatonin implants on reproduction and growth of turbot broodstock. Aquac. Int. 9, 477-487
-
- Amano M., Iigo M., Ikuta K., Kitamura S., Yamada H., Yamamori K. (2000). Roles of melatonin in gonadal maturation of underyearling precocious male masu salmon. Gen. Comp. Endocrinol. 120, 190-197 - PubMed
-
- Azpeleta C., Martínez-Alvarez R. M., Delgado M. J., Isorna E., De Pedro N. (2010). Melatonin reduces locomotor activity and circulating cortisol in goldfish. Horm. Behav. 57, 323-329 - PubMed
-
- Bandler R., Carrive P. (1988). Integrated defence reaction elicited by excitatory amino acid microinjection in the midbrain periaqueductal grey region of the unrestrained cat. Brain Res. 439, 95-106 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

