Green synthesis of silver nanoparticles using Ganoderma neo-japonicum Imazeki: a potential cytotoxic agent against breast cancer cells
- PMID: 24265551
- PMCID: PMC3833323
- DOI: 10.2147/IJN.S51881
Green synthesis of silver nanoparticles using Ganoderma neo-japonicum Imazeki: a potential cytotoxic agent against breast cancer cells
Abstract
Background: Silver nanoparticles (AgNPs) are an important class of nanomaterial for a wide range of industrial and biomedical applications. AgNPs have been used as antimicrobial and disinfectant agents due their detrimental effect on target cells. The aim of our study was to determine the cytotoxic effects of biologically synthesized AgNPs using hot aqueous extracts of the mycelia of Ganoderma neo-japonicum Imazeki on MDA-MB-231 human breast cancer cells.
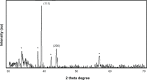
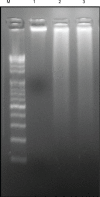
Methods: We developed a green method for the synthesis of water-soluble AgNPs by treating silver ions with hot aqueous extract of the mycelia of G. neo-japonicum. The formation of AgNPs was characterized by ultraviolet-visible absorption spectroscopy, X-ray diffraction, dynamic light scattering, and transmission electron microscopy. Furthermore, the toxicity of synthesized AgNPs was evaluated using a series of assays: such as cell viability, lactate dehydrogenase leakage, reactive oxygen species generation, caspase 3, DNA laddering, and terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling in human breast cancer cells (MDA-MB-231).
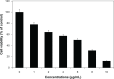
Results: The ultraviolet-visible absorption spectroscopy results showed a strong resonance centered on the surface of AgNPs at 420 nm. The X-ray diffraction analysis confirmed that the synthesized AgNPs were single-crystalline, corresponding with the result of transmission electron microscopy. Treatment of MDA-MB-231 breast cancer cells with various concentrations of AgNPs (1-10 μg/mL) for 24 hours revealed that AgNPs could inhibit cell viability and induce membrane leakage in a dose-dependent manner. Cells exposed to AgNPs showed increased reactive oxygen species and hydroxyl radical production. Furthermore, the apoptotic effects of AgNPs were confirmed by activation of caspase 3 and DNA nuclear fragmentation.
Conclusion: The results indicate that AgNPs possess cytotoxic effects with apoptotic features and suggest that the reactive oxygen species generated by AgNPs have a significant role in apoptosis. The present findings suggest that AgNPs could contribute to the development of a suitable anticancer drug, which may lead to the development of a novel nanomedicine for the treatment of cancers.
Keywords: AgNPs; DNA fragmentation; Ganoderma neo-japonicum; caspase-3 activity; cytotoxicity; human breast cancer cells.
Figures








Similar articles
-
Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 human breast cancer cells: targeting p53 for anticancer therapy.Int J Nanomedicine. 2015 Jun 29;10:4203-22. doi: 10.2147/IJN.S83953. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26170659 Free PMC article.
-
Cytotoxicity of biologically synthesized silver nanoparticles in MDA-MB-231 human breast cancer cells.Biomed Res Int. 2013;2013:535796. doi: 10.1155/2013/535796. Epub 2013 Jul 8. Biomed Res Int. 2013. PMID: 23936814 Free PMC article.
-
Insight into the molecular mechanism, cytotoxic, and anticancer activities of phyto-reduced silver nanoparticles in MCF-7 breast cancer cell lines.Microsc Res Tech. 2024 Jul;87(7):1627-1639. doi: 10.1002/jemt.24540. Epub 2024 Mar 7. Microsc Res Tech. 2024. PMID: 38450823
-
Bioinspired and Green Synthesis of Silver Nanoparticles for Medical Applications: A Green Perspective.Appl Biochem Biotechnol. 2024 Jun;196(6):3636-3669. doi: 10.1007/s12010-023-04719-z. Epub 2023 Sep 5. Appl Biochem Biotechnol. 2024. PMID: 37668757 Free PMC article. Review.
-
Pharmaceutical Aspects of Green Synthesized Silver Nanoparticles: A Boon to Cancer Treatment.Anticancer Agents Med Chem. 2021;21(12):1490-1509. doi: 10.2174/1871520620666200918111024. Anticancer Agents Med Chem. 2021. PMID: 32951580 Review.
Cited by
-
Phytochemicals and Biogenic Metallic Nanoparticles as Anticancer Agents.Oxid Med Cell Longev. 2016;2016:3685671. doi: 10.1155/2016/3685671. Epub 2016 Feb 23. Oxid Med Cell Longev. 2016. PMID: 27057273 Free PMC article. Review.
-
"Therapeutic advancements in nanomedicine: The multifaceted roles of silver nanoparticles".Biotechnol Notes. 2024 Jun 1;5:64-79. doi: 10.1016/j.biotno.2024.05.002. eCollection 2024. Biotechnol Notes. 2024. PMID: 39416696 Free PMC article. Review.
-
Plant-based metallic nanoparticles as potential theranostics agents: bioinspired tool for imaging and treatment.IET Nanobiotechnol. 2018 Oct;12(7):869-878. doi: 10.1049/iet-nbt.2017.0325. IET Nanobiotechnol. 2018. PMID: 30247124 Free PMC article. Review.
-
Biogenic synthesis of silver nanoparticles: Antibacterial and cytotoxic potential.Saudi J Biol Sci. 2020 May;27(5):1340-1351. doi: 10.1016/j.sjbs.2019.12.014. Epub 2019 Dec 19. Saudi J Biol Sci. 2020. PMID: 32346344 Free PMC article.
-
Acalypha indica Linn: Biogenic synthesis of silver and gold nanoparticles and their cytotoxic effects against MDA-MB-231, human breast cancer cells.Biotechnol Rep (Amst). 2014 Aug 13;4:42-49. doi: 10.1016/j.btre.2014.08.002. eCollection 2014 Dec. Biotechnol Rep (Amst). 2014. PMID: 28626661 Free PMC article.
References
-
- Chen X, Schluesener HJ. Nanosilver: a nanoproduct in medical application. Toxicol Lett. 2008;176:1–12. - PubMed
-
- Tian J, Wong KK, Ho CM, et al. Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem. 2007;2:129–136. - PubMed
-
- Malik MA, Brien PO, Revaprasadu N. A simple route to the synthesis of core/shell nanoparticles of chalcogenides. Chem Mater. 2002;14:2004–2010.
-
- Thakkar KN, Mhatre SS, Parikh RY. Biological synthesis of metallic nanoparticles. Nanomedicine. 2010;6:257–262. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

