Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection
- PMID: 24265599
- PMCID: PMC3825655
- DOI: 10.1371/journal.pmed.1001549
Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection
Abstract
Background: Respiratory syncytial virus (RSV) is the leading cause of viral lower respiratory tract infection (LRTI) and hospitalization in infants. Mostly because of the incomplete understanding of the disease pathogenesis, there is no licensed vaccine, and treatment remains symptomatic. We analyzed whole blood transcriptional profiles to characterize the global host immune response to acute RSV LRTI in infants, to characterize its specificity compared with influenza and human rhinovirus (HRV) LRTI, and to identify biomarkers that can objectively assess RSV disease severity.
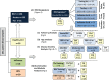
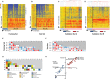
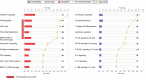
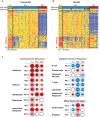
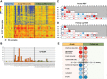
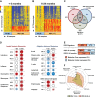
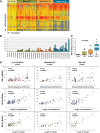
Methods and findings: This was a prospective observational study over six respiratory seasons including a cohort of infants hospitalized with RSV (n = 135), HRV (n = 30), and influenza (n = 16) LRTI, and healthy age- and sex-matched controls (n = 39). A specific RSV transcriptional profile was identified in whole blood (training cohort, n = 45 infants; Dallas, Texas, US) and validated in three different cohorts (test cohort, n = 46, Dallas, Texas, US; validation cohort A, n = 16, Turku, Finland; validation cohort B, n = 28, Columbus, Ohio, US) with high sensitivity (94% [95% CI 87%-98%]) and specificity (98% [95% CI 88%-99%]). It classified infants with RSV LRTI versus HRV or influenza LRTI with 95% accuracy. The immune dysregulation induced by RSV (overexpression of neutrophil, inflammation, and interferon genes, and suppression of T and B cell genes) persisted beyond the acute disease, and immune dysregulation was greatly impaired in younger infants (<6 mo). We identified a genomic score that significantly correlated with outcomes of care including a clinical disease severity score and, more importantly, length of hospitalization and duration of supplemental O2.
Conclusions: Blood RNA profiles of infants with RSV LRTI allow specific diagnosis, better understanding of disease pathogenesis, and assessment of disease severity. This study opens new avenues for biomarker discovery and identification of potential therapeutic or preventive targets, and demonstrates that large microarray datasets can be translated into a biologically meaningful context and applied to the clinical setting. Please see later in the article for the Editors' Summary.
Conflict of interest statement
In the last 3 years, OR has had financial relations with companies that are involved with respiratory viruses research or product as follows: Advisory boards: Gilead, Abbvie, Alios, Quidel; Honoraria for Lectures and Co-Chair Medical Conferences: Abbvie; Cover part of travel expenses to present clinical study at a scientific conference: MedImmune; Research Grant: Abbott Molecular. In the last 3 years, AM has had relations with companies that are involved with respiratory viruses research or product as follows: Advisory Boards: Alios, Janssen Infectious Diseases BVBA; Honoraria for Lectures at CME Conferences: Abbvie; Research Grant: Gilead. All other authors have declared that no competing interest exist.
Figures


References
-
- Bryce J, Boschi-Pinto C, Shibuya K, Black RE (2005) WHO estimates of the causes of death in children. Lancet 365: 1147–1152. - PubMed
-
- Mulholland K, Hilton S, Adegbola R, Usen S, Oparaugo A, et al. (1997) Randomised trial of Haemophilus influenzae type-b tetanus protein conjugate vaccine [corrected] for prevention of pneumonia and meningitis in Gambian infants. Lancet 349: 1191–1197. - PubMed
-
- Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, et al. (2003) A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med 349: 1341–1348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

