Increasing the yield in targeted next-generation sequencing by implicating CNV analysis, non-coding exons and the overall variant load: the example of retinal dystrophies
- PMID: 24265693
- PMCID: PMC3827063
- DOI: 10.1371/journal.pone.0078496
Increasing the yield in targeted next-generation sequencing by implicating CNV analysis, non-coding exons and the overall variant load: the example of retinal dystrophies
Erratum in
- PLoS One. 2014;9(11):e108840
Abstract
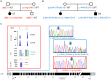
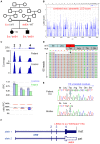
Retinitis pigmentosa (RP) and Leber congenital amaurosis (LCA) are major causes of blindness. They result from mutations in many genes which has long hampered comprehensive genetic analysis. Recently, targeted next-generation sequencing (NGS) has proven useful to overcome this limitation. To uncover "hidden mutations" such as copy number variations (CNVs) and mutations in non-coding regions, we extended the use of NGS data by quantitative readout for the exons of 55 RP and LCA genes in 126 patients, and by including non-coding 5' exons. We detected several causative CNVs which were key to the diagnosis in hitherto unsolved constellations, e.g. hemizygous point mutations in consanguineous families, and CNVs complemented apparently monoallelic recessive alleles. Mutations of non-coding exon 1 of EYS revealed its contribution to disease. In view of the high carrier frequency for retinal disease gene mutations in the general population, we considered the overall variant load in each patient to assess if a mutation was causative or reflected accidental carriership in patients with mutations in several genes or with single recessive alleles. For example, truncating mutations in RP1, a gene implicated in both recessive and dominant RP, were causative in biallelic constellations, unrelated to disease when heterozygous on a biallelic mutation background of another gene, or even non-pathogenic if close to the C-terminus. Patients with mutations in several loci were common, but without evidence for di- or oligogenic inheritance. Although the number of targeted genes was low compared to previous studies, the mutation detection rate was highest (70%) which likely results from completeness and depth of coverage, and quantitative data analysis. CNV analysis should routinely be applied in targeted NGS, and mutations in non-coding exons give reason to systematically include 5'-UTRs in disease gene or exome panels. Consideration of all variants is indispensable because even truncating mutations may be misleading.
Conflict of interest statement
Figures


References
-
- Wright AF, Chakarova CF, Abd El-Aziz MM, Bhattacharya SS (2010) Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat Rev Genet 11: 273–284. - PubMed
-
- Frick KD, Roebuck MC, Feldstein JI, McCarty CA, Grover LL (2012) Health services utilization and cost of retinitis pigmentosa. Arch Ophthalmol 130: 629–634. - PubMed
-
- Hartong DT, Berson EL, Dryja TP (2006) Retinitis pigmentosa. Lancet 368: 1795–1809. - PubMed
-
- Sheffield VC, Stone EM (2011) Genomics and the eye. N Engl J Med 364: 1932–1942. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

