Ex-vivo tolerogenic F4/80⁺ antigen-presenting cells (APC) induce efferent CD8⁺ regulatory T cell-dependent suppression of experimental autoimmune uveitis
- PMID: 24266626
- PMCID: PMC3958152
- DOI: 10.1111/cei.12243
Ex-vivo tolerogenic F4/80⁺ antigen-presenting cells (APC) induce efferent CD8⁺ regulatory T cell-dependent suppression of experimental autoimmune uveitis
Abstract
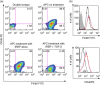
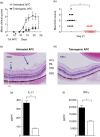
It is known that inoculation of antigen into the anterior chamber (a.c.) of a mouse eye induces a.c.-associated immune deviation (ACAID), which is mediated in part by antigen-specific local and peripheral tolerance to the inciting antigen. ACAID can also be induced in vivo by intravenous (i.v.) inoculation of ex-vivo-generated tolerogenic antigen-presenting cells (TolAPC). The purpose of this study was to test if in-vitro-generated retinal antigen-pulsed TolAPC suppressed established experimental autoimmune uveitis (EAU). Retinal antigen-pulsed TolAPC were injected i.v. into mice 7 days post-induction of EAU. We observed that retinal antigen-pulsed TolAPC suppressed the incidence and severity of the clinical expression of EAU and reduced the expression of associated inflammatory cytokines. Moreover, extract of whole retina efficiently replaced interphotoreceptor retinoid-binding protein (IRBP) in the preparation of TolAPC used to induce tolerance in EAU mice. Finally, the suppression of EAU could be transferred to a new set of EAU mice with CD8⁺ but not with CD4⁺ regulatory T cells (T(reg)). Retinal antigen-pulsed TolAPC suppressed ongoing EAU by inducing CD8⁺ T(reg) cells that, in turn, suppressed the effector activity of the IRBP-specific T cells and altered the clinical symptoms of autoimmune inflammation in the eye. The ability to use retinal extract for the antigen raises the possibility that retinal extract could be used to produce autologous TolAPC and then used as therapy in human uveitis.
Keywords: ACAID; CD8+ Treg; EAU; autoimmunity; tolerogenic APC.
© 2013 The Authors. Clinical and Experimental Immunology published by John Wiley & Sons Ltd on behalf of British Society. for Immunology.
Figures



References
-
- Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:878–889. - PubMed
-
- Taylor AW. Neuropeptides, aqueous humor, and ocular immune privilege. In: Troger J, Kieslbach G, Bechrakis N, editors. Neuropeptides in the eye. Kerala: Research Signpost; 2009. pp. 79–91.
-
- Hori J, Wang M, Miyashita M, et al. B7-H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J Immunol. 2006;177:5928–5935. - PubMed
-
- Ferguson TA, Griffith TS. The role of Fas ligand and TNF-related apoptosis-inducing ligand (TRAIL) in the ocular immune response. Chem Immunol Allergy. 2007;92:140–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

