Endoscopic ulcers as a surrogate marker of NSAID-induced mucosal damage
- PMID: 24267380
- PMCID: PMC3891314
- DOI: 10.1186/ar4176
Endoscopic ulcers as a surrogate marker of NSAID-induced mucosal damage
Abstract
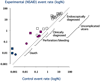
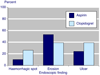
The characteristic of a biomarker that makes it a useful surrogate is the ability to identify a high risk of clinically important benefits or harms occurring in the future. A number of definitions or descriptions of surrogate definition have been put forward. Most recently the Institute of Medicine of the National Academy of Sciences in the USA has put forward an evaluation scheme for biomarkers, looking at validation (assay performance), qualification (assessment of evidence), and utilisation (the context in which the surrogate is to be used). This paper examines the example of endoscopy as a surrogate marker of NSAID-induced mucosal damage using the Institute of Medicine criteria. The article finds extensive evidence that the detection of endoscopic ulcers is a valid marker. The process of qualification documents abundant evidence showing that endoscopic ulcers and serious upper gastrointestinal damage are influenced in the same direction and much the same magnitude by a variety of risk factors and interventions. Criticisms of validation and qualification for endoscopic ulcers have been examined, and dismissed. Context is the key, and in the context of serious NSAID-induced upper gastrointestinal harm, endoscopic ulcers represent a useful surrogate. Generalisability beyond this context is not considered.
Figures





References
-
- Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;15:1383–1389. - PubMed
-
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharm Ther. 2001;15:89–95. - PubMed
-
- Institute of Medicine. Evaluation of Biomarkers and Surrogate Endpoints in Chronic Disease. Washington, DC: The National Academies Press; 2010. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

