Drosophila melanogaster as a model organism for Alzheimer's disease
- PMID: 24267573
- PMCID: PMC4222597
- DOI: 10.1186/1750-1326-8-35
Drosophila melanogaster as a model organism for Alzheimer's disease
Abstract
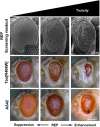
Drosophila melanogaster provides an important resource for in vivo modifier screens of neurodegenerative diseases. To study the underlying pathogenesis of Alzheimer's disease, fly models that address Tau or amyloid toxicity have been developed. Overexpression of human wild-type or mutant Tau causes age-dependent neurodegeneration, axonal transport defects and early death. Large-scale screens utilizing a neurodegenerative phenotype induced by eye-specific overexpression of human Tau have identified several kinases and phosphatases, apoptotic regulators and cytoskeleton proteins as determinants of Tau toxicity in vivo. The APP ortholog of Drosophila (dAPPl) shares the characteristic domains with vertebrate APP family members, but does not contain the human Aβ42 domain. To circumvent this drawback, researches have developed strategies by either direct secretion of human Aβ42 or triple transgenic flies expressing human APP, β-secretase and Drosophila γ-secretase presenilin (dPsn). Here, we provide a brief overview of how fly models of AD have contributed to our knowledge of the pathomechanisms of disease.
Figures

References
-
- Burdick D, Soreghan B, Kwon M, Kosmoski J, Knauer M, Henschen A, Yates J, Cotman C, Glabe C. Assembly and aggregation properties of synthetic Alzheimer’s A4/beta amyloid peptide analogs. J Biol Chem. 1992;267:546–554. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

