Single-neuron correlates of atypical face processing in autism
- PMID: 24267649
- PMCID: PMC3934412
- DOI: 10.1016/j.neuron.2013.08.029
Single-neuron correlates of atypical face processing in autism
Abstract
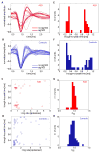
People with autism spectrum disorder (ASD) show abnormal processing of faces. A range of morphometric, histological, and neuroimaging studies suggest the hypothesis that this abnormality may be linked to the amygdala. We recorded data from single neurons within the amygdalae of two rare neurosurgical patients with ASD. While basic electrophysiological response parameters were normal, there were specific and striking abnormalities in how individual facial features drove neuronal response. Compared to control patients, a population of neurons in the two ASD patients responded significantly more to the mouth, but less to the eyes. Moreover, we found a second class of face-responsive neurons for which responses to faces appeared normal. The findings confirm the amygdala's pivotal role in abnormal face processing by people with ASD at the cellular level and suggest that dysfunction may be traced to a specific subpopulation of neurons with altered selectivity for the features of faces.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

