Heart-specific stiffening in early embryos parallels matrix and myosin expression to optimize beating
- PMID: 24268417
- PMCID: PMC4116639
- DOI: 10.1016/j.cub.2013.10.057
Heart-specific stiffening in early embryos parallels matrix and myosin expression to optimize beating
Abstract
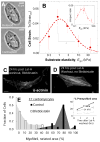
In development and differentiation, morphological changes often accompany mechanical changes [1], but it is unclear whether or when cells in embryos sense tissue elasticity. The earliest embryo is uniformly pliable, while adult tissues vary widely in mechanics from soft brain and stiff heart to rigid bone [2]. However, cell sensitivity to microenvironment elasticity is debated based in part on results from complex three-dimensional culture models [3]. Regenerative cardiology provides strong motivation to clarify any cell-level sensitivities to tissue elasticity because rigid postinfarct regions limit pumping by the adult heart [4]. Here, we focus on the spontaneously beating embryonic heart and sparsely cultured cardiomyocytes, including cells derived from pluripotent stem cells. Tissue elasticity, Et, increases daily for heart to 1-2 kPa by embryonic day 4 (E4), and although this is ~10-fold softer than adult heart, the beating contractions of E4 cardiomyocytes prove optimal at ~Et,E4 both in vivo and in vitro. Proteomics reveals daily increases in a small subset of proteins, namely collagen plus cardiac-specific excitation-contraction proteins. Rapid softening of the heart's matrix with collagenase or stiffening it with enzymatic crosslinking suppresses beating. Sparsely cultured E4 cardiomyocytes on collagen-coated gels likewise show maximal contraction on matrices with native E4 stiffness, highlighting cell-intrinsic mechanosensitivity. While an optimal elasticity for striation proves consistent with the mathematics of force-driven sarcomere registration, contraction wave speed is linear in Et as theorized for excitation-contraction coupled to matrix elasticity. Pluripotent stem cell-derived cardiomyocytes also prove to be mechanosensitive to matrix and thus generalize the main observation that myosin II organization and contractile function are optimally matched to the load contributed by matrix elasticity.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures



References
-
- Gonzalez-Rodriguez D, Guevorkian K, Douezan S, Brochard-Wyart F. Soft matter models of developing tissues and tumors. Science. 2012;338(6109):910–917. - PubMed
-
- Chien K, Domian I, Parker K. Cardiogenesis and the complex biology of regenerative cardiovascular medicine. Science. 2008;322(5907):1494–1497. - PubMed
-
- Butcher JT, McQuinn TC, Sedmera D, Turner D, Markwald RR. Transitions in early embryonic atrioventricular valvular function correspond with changes in cushion biomechanics that are predictable by tissue composition. Integrative Physiology. 2007;100:1503–1511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 EB004000/EB/NIBIB NIH HHS/United States
- R01 HL062352/HL/NHLBI NIH HHS/United States
- 8UL1TR000003/TR/NCATS NIH HHS/United States
- R01 EB007049/EB/NIBIB NIH HHS/United States
- R21 AI087516/AI/NIAID NIH HHS/United States
- R01HL062352/HL/NHLBI NIH HHS/United States
- S10 RR022575/RR/NCRR NIH HHS/United States
- P01 DK032094/DK/NIDDK NIH HHS/United States
- R21 EB004489/EB/NIBIB NIH HHS/United States
- P01DK032094/DK/NIDDK NIH HHS/United States
- R21 AR056128/AR/NIAMS NIH HHS/United States
- R21 EB003164/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

