Amyloid burden and neural function in people at risk for Alzheimer's Disease
- PMID: 24269021
- PMCID: PMC4018215
- DOI: 10.1016/j.neurobiolaging.2013.09.028
Amyloid burden and neural function in people at risk for Alzheimer's Disease
Abstract
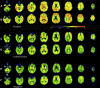
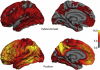
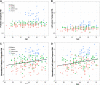
To determine the relationship between amyloid burden and neural function in healthy adults at risk for Alzheimer's Disease (AD), we used multimodal imaging with [C-11]Pittsburgh compound B positron emission tomography, [F-18]fluorodeoxyglucose, positron emission tomography , and magnetic resonance imaging, together with cognitive measurement in 201 subjects (mean age, 60.1 years; range, 46-73 years) from the Wisconsin Registry for Alzheimer's Prevention. Using a qualitative rating, 18% of the samples were strongly positive Beta-amyloid (Aβ+), 41% indeterminate (Aβi), and 41% negative (Aβ-). Aβ+ was associated with older age, female sex, and showed trends for maternal family history of AD and APOE4. Relative to the Aβ- group, Aβ+ and Aβi participants had increased glucose metabolism in the bilateral thalamus; Aβ+ participants also had increased metabolism in the bilateral superior temporal gyrus. Aβ+ participants exhibited increased gray matter in the lateral parietal lobe bilaterally relative to the Aβ- group, and no areas of significant atrophy. Cognitive performance and self report cognitive and affective symptoms did not differ between groups. Amyloid burden can be identified in adults at a mean age of 60 years and is accompanied by glucometabolic increases in specific areas, but not atrophy or cognitive loss. This asymptomatic stage may be an opportune window for intervention to prevent progression to symptomatic AD.
Keywords: AD risk; Alzheimer's disease; Amyloid imaging; Cognitive function; Glucose metabolism.
Published by Elsevier Inc.
Figures
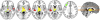
References
-
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. - PMC - PubMed
-
- Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. - PubMed
-
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N. Engl. J. Med. 2012;367:795–804. http://dx.doi.org/10.1056/NEJMoa1202753. - DOI - PMC - PubMed
-
- Bendlin BB, Ries ML, Canu E, Sodhi A, Lazar M, Alexander AL, Carlsson CM, Sager MA, Asthana S, Johnson SC. White matter is altered with parental family history of Alzheimer's disease. Alzheimers Dement. 2010;6:394–403. http://dx.doi.org/10.1016/j.jalz.2009.11.003. - DOI - PMC - PubMed
-
- Chetelat G. Alzheimer disease: Abeta-independent processes-rethinking preclinical AD. Nat. Rev. Neurol. 2013;9:123–124. http://dx.doi.org/10.1038/nrneurol.2013.21. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 HD003352/HD/NICHD NIH HHS/United States
- UL1 TR000427/TR/NCATS NIH HHS/United States
- AG027161/AG/NIA NIH HHS/United States
- P50 HD03352/HD/NICHD NIH HHS/United States
- I01 CX000165/CX/CSRD VA/United States
- R01 AG027161/AG/NIA NIH HHS/United States
- T32 EB013180/EB/NIBIB NIH HHS/United States
- UL1 TR000457/TR/NCATS NIH HHS/United States
- UL1 RR025011/RR/NCRR NIH HHS/United States
- R01 AG021155/AG/NIA NIH HHS/United States
- AG021155/AG/NIA NIH HHS/United States
- RF1 AG027161/AG/NIA NIH HHS/United States
- UL1RR025011/RR/NCRR NIH HHS/United States
- P50 AG033514/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

