Glycotherapy: new advances inspire a reemergence of glycans in medicine
- PMID: 24269151
- PMCID: PMC4111574
- DOI: 10.1016/j.chembiol.2013.09.010
Glycotherapy: new advances inspire a reemergence of glycans in medicine
Abstract
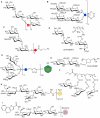
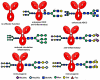
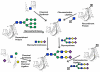
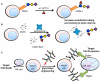
The beginning of the 20(th) century marked the dawn of modern medicine with glycan-based therapies at the forefront. However, glycans quickly became overshadowed as DNA- and protein-focused treatments became readily accessible. The recent development of new tools and techniques to study and produce structurally defined carbohydrates has spurred renewed interest in the therapeutic applications of glycans. This review focuses on advances within the past decade that are bringing glycan-based treatments back to the forefront of medicine and the technologies that are driving these efforts. These include the use of glycans themselves as therapeutic molecules as well as engineering protein and cell surface glycans to suit clinical applications. Glycan therapeutics offer a rich and promising frontier for developments in the academic, biopharmaceutical, and medical fields.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
Breakthrough of glycobiology in the 21st century.Front Immunol. 2023 Jan 5;13:1071360. doi: 10.3389/fimmu.2022.1071360. eCollection 2022. Front Immunol. 2023. PMID: 36685548 Free PMC article. Review.
-
Proteoglycans in Biomedicine: Resurgence of an Underexploited Class of ECM Molecules.Front Pharmacol. 2020 Jan 29;10:1661. doi: 10.3389/fphar.2019.01661. eCollection 2019. Front Pharmacol. 2020. PMID: 32082161 Free PMC article. Review.
-
Expediting Glycospace Exploration: Therapeutic Glycans via Automated Synthesis.Angew Chem Int Ed Engl. 2025 Mar 24;64(13):e202422766. doi: 10.1002/anie.202422766. Epub 2025 Feb 21. Angew Chem Int Ed Engl. 2025. PMID: 39936247 Free PMC article. Review.
-
Bacterial glycobiotechnology: A biosynthetic route for the production of biopharmaceutical glycans.Biotechnol Adv. 2023 Oct;67:108180. doi: 10.1016/j.biotechadv.2023.108180. Epub 2023 May 24. Biotechnol Adv. 2023. PMID: 37236328 Review.
-
Advances in cell surface glycoengineering reveal biological function.Glycobiology. 2016 Aug;26(8):789-96. doi: 10.1093/glycob/cww045. Epub 2016 Apr 10. Glycobiology. 2016. PMID: 27066802 Free PMC article. Review.
Cited by
-
Efficient O-Functionalization of Carbohydrates with Electrophilic Reagents.Angew Chem Int Ed Engl. 2016 Sep 5;55(37):11226-30. doi: 10.1002/anie.201605999. Epub 2016 Aug 16. Angew Chem Int Ed Engl. 2016. PMID: 27528184 Free PMC article.
-
Label-Free Detection of Glycan-Protein Interactions for Array Development by Surface-Enhanced Raman Spectroscopy (SERS).Chemistry. 2016 Aug 1;22(32):11180-11185. doi: 10.1002/chem.201602706. Epub 2016 Jun 30. Chemistry. 2016. PMID: 27304194 Free PMC article.
-
Thioglycosides Are Efficient Metabolic Decoys of Glycosylation that Reduce Selectin Dependent Leukocyte Adhesion.Cell Chem Biol. 2018 Dec 20;25(12):1519-1532.e5. doi: 10.1016/j.chembiol.2018.09.012. Epub 2018 Oct 18. Cell Chem Biol. 2018. PMID: 30344053 Free PMC article.
-
Glycan Mimetics from Natural Products: New Therapeutic Opportunities for Neurodegenerative Disease.Molecules. 2019 Dec 16;24(24):4604. doi: 10.3390/molecules24244604. Molecules. 2019. PMID: 31888221 Free PMC article. Review.
-
Use of Mass Spectrometry to Screen Glycan Early Markers in Hepatocellular Carcinoma.Front Oncol. 2018 Jan 15;7:328. doi: 10.3389/fonc.2017.00328. eCollection 2017. Front Oncol. 2018. PMID: 29379771 Free PMC article. Review.
References
-
- Abian O, Alfonso P, Velazquez-Campoy A, Giraldo P, Pocovi M, Sancho J. Therapeutic strategies for Gaucher disease: miglustat (NB-DNJ) as a pharmacological chaperone for glucocerebrosidase and the different thermostability of velaglucerase alfa and imiglucerase. Mol. Pharm. 2011;8:2390–2397. - PubMed
-
- Ametamey SM, Honer M, Schubiger PA. Molecular imaging with PET. Chem. Rev. 2008;108:1501–1516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

