Reduced-intensity allogeneic transplantation using alemtuzumab from HLA-matched related, unrelated, or haploidentical related donors for patients with hematologic malignancies
- PMID: 24269380
- PMCID: PMC4140655
- DOI: 10.1016/j.bbmt.2013.11.010
Reduced-intensity allogeneic transplantation using alemtuzumab from HLA-matched related, unrelated, or haploidentical related donors for patients with hematologic malignancies
Abstract
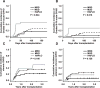
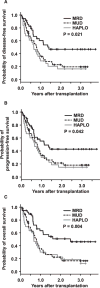
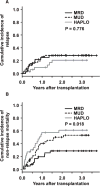
We present a comparative study on 124 patients with hematologic malignancies who had undergone reduced-intensity conditioning and then received a transplant from an HLA-matched related (MRD), an HLA-matched unrelated (MUD), or an HLA-haploidentical related (HAPLO) donor. The conditioning regimen, which consisted of fludarabine, melphalan or busulfan, and alemtuzumab was administered to patients with lymphoid (n = 62) or myeloid disease (n = 62). Mycophenolate mofetil was used as prophylaxis for graft-versus-host disease (GVHD), and 38, 58, and 33 patients received transplants from MRD, MUD, and HAPLO donors, respectively. Only 2 patients experienced primary graft failure (GF) after melphalan-based regimen, whereas 8 of the 17 patients who received a transplant from HAPLO donors experienced a primary GF after busulfan-based regimen. The cumulative incidence of grade III to IV acute GVHD in engrafted patients who had received transplants from MRD, MUD, or HAPLO donors was 3%, 11%, and 27%, respectively, and the 2-year overall survival (OS) rates were 51%, 22%, and 23%, respectively. According to multivariate analysis, transplantation from either MUD or HAPLO donors compared with MRD were adverse factors that affected the OS (P = .006 and P = .002, respectively). In conclusion, the reduced-intensity regimen that included fludarabine, busulfan, or melphalan and alemtuzumab using only mycophenolate mofetil as the GVHD prophylaxis conferred favorable outcomes in the MRD group but lower survival rates in the MUD and HAPLO groups. The busulfan-based regimen led to a high incidence of GF in the HAPLO group, suggesting the need for modification or intensification of immunosuppression.
Keywords: Alemtuzumab; Haploidentical; Matched-related; Matched-unrelated; Reduced-intensity.
Copyright © 2014 American Society for Blood and Marrow Transplantation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

References
-
- Horwitz ME. Reduced intensity versus myeloablative allogeneic stem cell transplantation for the treatment of acute myeloid leukemia, myelodysplastic syndrome and acute lymphoid leukemia. Curr Opin Oncol. 2011;23:197–202. - PubMed
-
- Bornhauser M, Kienast J, Trenschel R, et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012;13:1035–1044. - PubMed
-
- Rizzieri DA, Long GD, Vredenburgh JJ, et al. Successful allogeneic engraftment of mismatched unrelated cord blood following a nonmyeloablative preparative regimen. Blood (ASH annual Meeting Abstracts) 2001;98:2486–3488. - PubMed
-
- Chakraverty R, Orti G, Roughton M, et al. Impact of in vivo alemtuzumab dose before reduced intensity conditioning and HLA-identical sibling stem cell transplantation: pharmacokinetics, GVHD, and immune reconstitution. Blood. 2010;116:3080–3088. - PubMed
-
- von dem Borne PA, Starrenburg CW, Halkes SJ, et al. Reduced-intensity conditioning allogeneic stem cell transplantation with donor T-cell depletion using alemtuzumab added to the graft (‘Campath in the bag’) Curr Opin Oncol. 2009;21(Suppl 1):S27–S29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

