Cell-based antiviral screening against coronaviruses: developing virus-specific and broad-spectrum inhibitors
- PMID: 24269477
- PMCID: PMC3931262
- DOI: 10.1016/j.antiviral.2013.11.004
Cell-based antiviral screening against coronaviruses: developing virus-specific and broad-spectrum inhibitors
Abstract
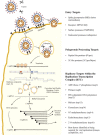
To combat the public health threat from emerging coronaviruses (CoV), the development of antiviral therapies with either virus-specific or pan-coronaviral activities is necessary. An important step in antiviral drug development is the screening of potential inhibitors in cell-based systems. The recent emergence of Middle East respiratory syndrome coronavirus (MERS-CoV) necessitates adapting methods that have been used to identify antivirals against severe acute respiratory syndrome coronavirus (SARS-CoV) and developing new approaches to more efficiently screen antiviral drugs. In this article we review cell-based assays using infectious virus (BSL-3) and surrogate assays (BSL-2) that can be implemented to accelerate antiviral development against MERS-CoV and future emergent coronaviruses. This paper forms part of a series of invited articles in Antiviral Research on "From SARS to MERS: 10years of research on highly pathogenic human coronaviruses."
Keywords: Antivirals; Entry inhibitors; MERS-CoV; Replicase inhibitors; SARS-CoV; pGlo.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
A safe and convenient pseudovirus-based inhibition assay to detect neutralizing antibodies and screen for viral entry inhibitors against the novel human coronavirus MERS-CoV.Virol J. 2013 Aug 26;10:266. doi: 10.1186/1743-422X-10-266. Virol J. 2013. PMID: 23978242 Free PMC article.
-
High-Throughput Screening and Identification of Potent Broad-Spectrum Inhibitors of Coronaviruses.J Virol. 2019 May 29;93(12):e00023-19. doi: 10.1128/JVI.00023-19. Print 2019 Jun 15. J Virol. 2019. PMID: 30918074 Free PMC article.
-
Assessing activity and inhibition of Middle East respiratory syndrome coronavirus papain-like and 3C-like proteases using luciferase-based biosensors.J Virol. 2013 Nov;87(21):11955-62. doi: 10.1128/JVI.02105-13. Epub 2013 Aug 28. J Virol. 2013. PMID: 23986593 Free PMC article.
-
Antiviral drugs specific for coronaviruses in preclinical development.Curr Opin Virol. 2014 Oct;8:45-53. doi: 10.1016/j.coviro.2014.06.002. Epub 2014 Jul 2. Curr Opin Virol. 2014. PMID: 24997250 Free PMC article. Review.
-
From SARS to MERS: 10 years of research on highly pathogenic human coronaviruses.Antiviral Res. 2013 Oct;100(1):286-95. doi: 10.1016/j.antiviral.2013.08.015. Epub 2013 Sep 6. Antiviral Res. 2013. PMID: 24012996 Free PMC article. Review.
Cited by
-
Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease.Clin Microbiol Rev. 2015 Apr;28(2):465-522. doi: 10.1128/CMR.00102-14. Clin Microbiol Rev. 2015. PMID: 25810418 Free PMC article. Review.
-
Cyclophilin A and CD147: novel therapeutic targets for the treatment of COVID-19.Med Drug Discov. 2020 Sep;7:100056. doi: 10.1016/j.medidd.2020.100056. Epub 2020 Jul 16. Med Drug Discov. 2020. PMID: 32835213 Free PMC article. Review.
-
Reprint of: Coronavirus reverse genetic systems: infectious clones and replicons.Virus Res. 2014 Dec 19;194:67-75. doi: 10.1016/j.virusres.2014.09.006. Epub 2014 Sep 26. Virus Res. 2014. PMID: 25261606 Free PMC article.
-
Coronavirus Disease 2019-COVID-19.Clin Microbiol Rev. 2020 Jun 24;33(4):e00028-20. doi: 10.1128/CMR.00028-20. Print 2020 Sep 16. Clin Microbiol Rev. 2020. PMID: 32580969 Free PMC article. Review.
-
COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics.Hum Vaccin Immunother. 2020 Jun 2;16(6):1232-1238. doi: 10.1080/21645515.2020.1735227. Epub 2020 Mar 18. Hum Vaccin Immunother. 2020. PMID: 32186952 Free PMC article. Review.
References
-
- Adedeji A.O., Singh K., Calcaterra N.E., DeDiego M.L., Enjuanes L., Weiss S., Sarafianos S.G. Severe acute respiratory syndrome coronavirus replication inhibitor that interferes with the nucleic acid unwinding of the viral helicase. Antimicrobial Agents and Chemotherapy. 2012;56(9):4718–4728. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

