Rotenone and paraquat perturb dopamine metabolism: A computational analysis of pesticide toxicity
- PMID: 24269752
- PMCID: PMC3893822
- DOI: 10.1016/j.tox.2013.11.003
Rotenone and paraquat perturb dopamine metabolism: A computational analysis of pesticide toxicity
Abstract
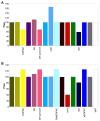
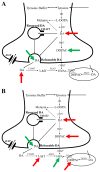
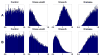
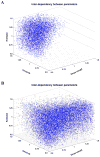
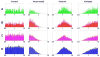
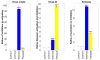
Pesticides, such as rotenone and paraquat, are suspected in the pathogenesis of Parkinson's disease (PD), whose hallmark is the progressive loss of dopaminergic neurons in the substantia nigra pars compacta. Thus, compounds expected to play a role in the pathogenesis of PD will likely impact the function of dopaminergic neurons. To explore the relationship between pesticide exposure and dopaminergic toxicity, we developed a custom-tailored mathematical model of dopamine metabolism and utilized it to infer potential mechanisms underlying the toxicity of rotenone and paraquat, asking how these pesticides perturb specific processes. We performed two types of analyses, which are conceptually different and complement each other. The first analysis, a purely algebraic reverse engineering approach, analytically and deterministically computes the altered profile of enzyme activities that characterize the effects of a pesticide. The second method consists of large-scale Monte Carlo simulations that statistically reveal possible mechanisms of pesticides. The results from the reverse engineering approach show that rotenone and paraquat exposures lead to distinctly different flux perturbations. Rotenone seems to affect all fluxes associated with dopamine compartmentalization, whereas paraquat exposure perturbs fluxes associated with dopamine and its breakdown metabolites. The statistical results of the Monte-Carlo analysis suggest several specific mechanisms. The findings are interesting, because no a priori assumptions are made regarding specific pesticide actions, and all parameters characterizing the processes in the dopamine model are treated in an unbiased manner. Our results show how approaches from computational systems biology can help identify mechanisms underlying the toxicity of pesticide exposure.
Keywords: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; 1-methyl-4-phenylpyridinium ion; 3,4-dihydroxyphenylacetate; Catsup; DAT; DOPAC; Dopamine; HVA; MPP+; MPTP; Mathematical model; Mode of action; ODEs; PD; Paraquat; Parkinson's disease; Rotenone; SNpc; catecholamines-up; dopamine transporter; homovanillic acid; ordinary differential equations; substantia nigra pars compacta.
Copyright © 2013 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Activation of c-Jun N-terminal protein kinase is a common mechanism underlying paraquat- and rotenone-induced dopaminergic cell apoptosis.Toxicol Sci. 2007 May;97(1):149-62. doi: 10.1093/toxsci/kfm029. Epub 2007 Feb 25. Toxicol Sci. 2007. PMID: 17324951
-
Divergent mechanisms of paraquat, MPP+, and rotenone toxicity: oxidation of thioredoxin and caspase-3 activation.Toxicol Sci. 2007 Jan;95(1):163-71. doi: 10.1093/toxsci/kfl125. Epub 2006 Oct 3. Toxicol Sci. 2007. PMID: 17018646
-
Paraquat neurotoxicity is distinct from that of MPTP and rotenone.Toxicol Sci. 2005 Nov;88(1):193-201. doi: 10.1093/toxsci/kfi304. Epub 2005 Sep 1. Toxicol Sci. 2005. PMID: 16141438
-
Paraquat- and rotenone-induced models of Parkinson's disease.Int J Immunopathol Pharmacol. 2011 Apr-Jun;24(2):313-22. doi: 10.1177/039463201102400205. Int J Immunopathol Pharmacol. 2011. PMID: 21658306 Review.
-
The neurotoxicity of pesticides: Implications for Parkinson's disease.Chemosphere. 2025 May;377:144348. doi: 10.1016/j.chemosphere.2025.144348. Epub 2025 Apr 9. Chemosphere. 2025. PMID: 40203643 Review.
Cited by
-
Parkinson's disease: Alterations in iron and redox biology as a key to unlock therapeutic strategies.Redox Biol. 2021 May;41:101896. doi: 10.1016/j.redox.2021.101896. Epub 2021 Feb 14. Redox Biol. 2021. PMID: 33799121 Free PMC article. Review.
-
SUR1 Receptor Interaction with Hesperidin and Linarin Predicts Possible Mechanisms of Action of Valeriana officinalis in Parkinson.Front Aging Neurosci. 2016 May 2;8:97. doi: 10.3389/fnagi.2016.00097. eCollection 2016. Front Aging Neurosci. 2016. PMID: 27199743 Free PMC article.
-
Comparison of the effect of rotenone and 1‑methyl‑4‑phenyl‑1,2,3,6‑tetrahydropyridine on inducing chronic Parkinson's disease in mouse models.Mol Med Rep. 2022 Mar;25(3):91. doi: 10.3892/mmr.2022.12607. Epub 2022 Jan 18. Mol Med Rep. 2022. PMID: 35039876 Free PMC article.
-
Strategies for Comparing Metabolic Profiles: Implications for the Inference of Biochemical Mechanisms from Metabolomics Data.IEEE/ACM Trans Comput Biol Bioinform. 2017 Nov-Dec;14(6):1434-1445. doi: 10.1109/TCBB.2016.2586065. Epub 2016 Jul 7. IEEE/ACM Trans Comput Biol Bioinform. 2017. PMID: 27392364 Free PMC article.
-
Identification of cancer mechanisms through computational systems modeling.Transl Cancer Res. 2014 Jun 1;3(3):233-242. doi: 10.3978/j.issn.2218-676X.2014.05.03. Transl Cancer Res. 2014. PMID: 26662197 Free PMC article.
References
-
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nature neuroscience. 2000;3:1301–1306. - PubMed
-
- Brooks AI, Chadwick CA, Gelbard HA, Cory-Slechta DA, Federoff HJ. Paraquat elicited neurobehavioral syndrome caused by dopaminergic neuron loss. Brain research. 1999;823:1–10. - PubMed
-
- Chiang AS, Lin CY, Chuang CC, Chang HM, Hsieh CH, Yeh CW, Shih CT, Wu JJ, Wang GT, Chen YC, Wu CC, Chen GY, Ching YT, Lee PC, Lin CY, Lin HH, Wu CC, Hsu HW, Huang YA, Chen JY, Chiang HJ, Lu CF, Ni RF, Yeh CY, Hwang JK. Three-dimensional reconstruction of brain-wide wiring networks in Drosophila at single-cell resolution. Current biology: CB. 2011;21:1–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

